Pakistan J. Zool., Vol. 50, Iss. 1, pp 75-82
Prospects of Entomopathogenic Bacteria and Fungi for Biological Control of Ricania simulans (Walker 1851) (Hemiptera: Ricaniidae)
Temel Gokturk1, Elif Tozlu2,* and Recep Kotan2
1Department of Forest Engineering, Forest Faculty, Artvin Coruh University, Artvin, Turkey
2Department of Plant Protection, Faculty of Agriculture, Atatürk University, Erzurum, Turkey
ABSTRACT
Ricania simulans causes harm in almost all plants that grow along the Eastern Black Sea coast. The chemicals used to control this pest are prohibited in this region due to tea cultivation. For this reason, new strategies are needed to control this pest. With the awareness on the negative effects of the chemicals used in the control against pests and with the increasing awareness on environmental issues, alternative methods were sought in the past; and in this context, studies were conducted to find new methods in which fungi and bacteria were used in the biological control against pests. Totally 10 bacterial strains including 2 strains of Brevibacillus brevis (CP-1, FD-1), 1 strain of Bacillus thuringiensis (FDP-1), 2 strains of Bacillus thuringiensis subsp. kenyae (FDP-8, FDP-42), 2 strains of Bacillus thuringiensis subsp. kurstakii (FDP-41, BAB-410), 1 strain of Bacillus subtilis (EK-7), 1 strain of Pseudomonas chlororaphis (NEM-28) and 1 strain of Bacillus sphaericus GC sub-group D (FD-49) and additionally 1 Beauveria bassiana (ET 10) fungus isolate were examined for their insecticidal activities in this study. The studied bio agents were tested by spraying on R. simulans nymphs and adults. B. thuringiensis subsp. kenyae, B. brevis and B. sphaericus GC subgroup D were the most effective on nymphs, whereas B. thuringiensis subsp. kurstakii, P. chlororaphi, and B. brevis were the most effective on adults. Under controlled conditions, mortality rate varied between 19.58%-42.08% in nymph applications, and between 6%-18% in adult applications.
Article Information
Received 24 April 2017
Revised 02 July 2017
Accepted 21 July 2017
Available online 26 December 2017
Authors’ Contribution
TG, ET and RK designed the study, each authors contributed equally to the study.
Key words
Bacteria, Beauveria bassiana, Biological control, Entomopathogens, Ricania simulans.
DOI: http://dx.doi.org/10.17582/journal.pjz/2018.50.1.75.82
* Corresponding author: elifalpertozlu@atauni.edu.tr
0030-9923/2018/0001-0075 $ 9.00/0
Copyright 2018 Zoological Society of Pakistan
Introductıon
Ricania simulans (Walker, 1851) (Hemiptera: Ricaniidae) is an important polyphagous pest that causes harm by sucking sap from hosts. Females lay their eggs under the skin of fresh sprouts and thin branches, causing death in these tissues over time (Gokturk and Aksu, 2014; Ak et al., 2015). The most common option for control against the R. simulans is chemical pest control. However, the region where this pest is common is reserved for tea cultivation, and also use of chemical pest control is prohibited in this region.
For this reason, recent efforts have focused on developing environmentally safe, long lasting and effective bio control methods for the management of pests. One method of biological pest control is the utilization of microbial agents for control against pests. Among bacterial bio insecticides, especially the spore-forming species belonging to the genus Bacillus, such as Bacillus popilliae, Bacillus thuringiensis and Bacillus sphaericus are commonly used in biological pest control. B. thuringiensis has been shown to be effective against some insects under the order Dipteran and Coleopteran, and particularly the harmful Lepidoptera larvae (Lacey et al., 2001).
Another group that is used in biological pest control is the fungi, and they are known to be entomopathogens against over 700 species belonging to 90 genera (Khachatourians and Sohail, 2008). Most of the entomopathogenic fungi are classified under the divisions of Ascomycota and Zygomycota. They have been effective in controlling many species of insects belonging to the genera Metarhizium, Beauveria, Trichoderma, Verticillium, Nomuraea, Entomophtora and Neozygites (Desphande, 1999). Among these, Beauveria isolates, and particularly Beauveria bassiana have been reported to be used in biological pest control against many harmful insect species, and their commercial preparations have been developed and introduced to the market (Inglis et al., 2001; Wraight et al., 2001; Vestergaad et al., 2003; Copping, 2004) and have minimum risk against natural enemies (Huang et al., 2012).
The present study was planned and conducted with the aim of determining and identifying entomopathogens effectively be used in biological control against R. simulans which continue to spread rapidly along the Eastern Black Sea coast and lead to economic losses.
Materıals and methods
Harmful insects, bacterial strains, fungal isolates and host plant
Different biological stages of R. simulans were obtained from the tea cultivation fields and kiwi gardens in Artvin and Rize provinces of Turkey. In this study, we obtained 100 bacterial and 100 fungal strains from adult and nymph stages of dead and diseased R. simulans. After 10 bacterial strains and 10 fungal isolates with different characteristics were chosen according to colony morphology and colour. Among these, only 4 bacterial strains were found to be effective, and the remaining 6 bacterial strains and 10 fungal isolates were grouped as other bacteria and fungi. In addition, 1 strain of Brevibacillus brevis (FD-1), 1 strain of Bacillus subtilis (EK-7), 2 strains of B. thuringiensis subsp. kenyae (FDP-8, FDP-42), 1 strain of B. thuringiensis subsp. kurstakii (FDP-41) and 1 strain of B. sphaericus GC subgroup D (FD-49) isolated from various diseased or dead insects in previous studies (Tozlu et al., 2011, 2016; Dadasoglu et al., 2013) and defined in the MIS and BIOLOG system based on fatty acid methyl esters and B. bassiana (ET 10) isolated from Sphenoptera antiqua (Illiger, 1803) larvae that are harmful to the trefoil plant (Onobrychis sativa L. (Fabacea)) were used in this study. Stock cultures of the bacterial strains and fungi isolate were kept in Atatürk University Faculty of Agriculture Plant Clinical Laboratory.
Tomato seedlings (Solanum lycopersicum L.) obtained from Artvin were used as host plants in order to determine the efficacy of bio agents studied in the laboratory.
Isolation of bacterial and fungal isolates
The diseased and dead nymphs and adults of R. simulans were subjected to surface sterilization by treating with 95% ethyl alcohol for 5 minutes. Then, cuticles of the nymphs were cut with a sterile scissor, and a drop of hemolymph was obtained and diluted in sterile water for inoculation (Thiery and Frachon, 1997). Adults were homogenized by pulverizing in a sterile mortar with sterile saline solution and serial dilutions were obtained from this homogenate. The dilutions prepared from the nymphs and adults were inoculated on Nutrient Agar (NA) and Tripticase Soy Agar (TSA) for bacterial growth and Water Agar (WA) and Potato Dextrose Agar (PDA) for fungal growth. The growth media were incubated at 30 °C, and at the end of 24-72 h. The colonies were purified according to different characteristics by transferring new growth media (Sezen and Demirbag, 2007). Each microorganism was given a separate code number, and information regarding the isolation conditions (location, altitude, insect form, date, etc.) were noted. Until the time of identification, the samples were kept at -86 °C in stock growth media containing 30% glycerol and Loria Broth (LB) for bacterial strains and in slant agar at +4 °C for fungal isolates
Identification of the bacteria by MIS and BIOLOG
The identity of all bacterial strains used in this study was confirmed according to fatty acid methyl esters (FAME) analysis by using Sherlock Microbial Identification System (Microbial ID, Newark, DE, USA) (Sasser, 1990).
The identity of the bacteria was confirmed according to BIOLOG systems. Two days before the inoculation of Biolog GP2 plates (Biolog), bacterial strains were streaked on Biolog Universal Growth Agar+25% Maltose agar plates. Each well of Biolog GP2 microtiter plates was inoculated with 125 µl of the bacterial suspension adjusted to the appropriate density (108 cfu/ml), and incubated at 27°C for 24 and 48 h. Colour development was automatically recorded using a microplate reader with a 590 nm wavelength filter. Identification results and similarity index of the bacteria was performed using BIOLOG420/Databases/GP601 (Holmes et al.,. 1994).
Hypersensitivity tests (HR) of the bacteria
The potential biocontrol bacterial strains were tested for hypersensitivity on tobacco plants (Nicotina tabacum L. var. Samsun) as described by Klement et al. (1964). The bacterial suspension (108 cfu/ml) prepared in sterile distilled water and infiltrated into inter costal area of the leaves of tobacco plants by using a 3-cc syringe without needle (Becton Dickinson, Franklin Lakes, NJ, USA). The inoculated plants were incubated in a completely randomized design on the greenhouse bench for 24–48 h at 20–28°C. The presence of rapid tissue necrosis at the inoculation site was recorded within 24–48 h after infiltration. This test was repeated at least three times for each strain. Sterilized distilled water (sdH2O) was used as a negative control.
Insecticidal effect of the bacteria under controlled conditions
Bacteria were grown in 50 ml flasks containing 20 mL of TSB medium on a rotary shaker at 27°C for 24 h. Absorbance of the bacterial suspensions was measured spectrophotometrically at 600 nm and appropriately diluted to 1x108 CFU/ml in sd.H2O.
B. bassiana ET 10 isolate was cultured on SDA with 1% yeast extract (SDAY) plates in several Petri dishes (9 cm in dia-meter), and were grown at 25 ± 1°C under a 16 h/8 h (light/dark) photoperiod and 60 ± 5% RH for fungal growth and conidial production. Surface of a 14-day-old culture was gently scratched with inoculation needle and transferred to vials containing 5 ml sterile Tween-80 solution (0.1% v/v). The concentration of conidia in stock suspensions were determined by direct count using hemocytometer. The conidial suspensions (106 conidia/ml) was prepared for the bioassay (Quesada et al., 2006).
Tomato seedlings were placed on pots, and the nymphs and/or adults collected 12-24 h ago were placed on each pot (20 on each) under cheesecloth, the prepared suspensions were sprayed over them. Plants were controlled daily and the number of dead adults and/or nymphs were noted. At the end of the experiment, number of dead adults and nymphs were used in the following formula to calculate the percent mortality rate:
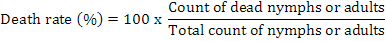
Pathogens from infected nymphs and adults were re-isolated according to Koch postulates; pathogenicity tests were repeated and their results were recorded. Sterile NB growth media used for diluting bacterial suspensions was used as a negative control and plant-origin pesticide Neemazal ® T/S containing the active substance Azadirachtin A, and pesticide Laser containing the active substance Spinosad were used as positive controls, these agents were previously tested against R.simulans and yielded successful results.
Analysis of results
All data in the present study were processed by JUMP 5.0 and the means were separated by LS Means Students tests. The statistical analyses of percentage values were performed by using transformed values.
Results
MIS and BIOLOG identification results and HR test results of 10 bacterial strains obtained from R. simulans were given Table I. According to BIOLOG identification results, CP-1 as Bacillus subtilis, FDP-1 and BAB-410 as Bacillus thuringiensis, NEM-28 as Pseudomonas sp. studied in this study as first were also confirmed by MIS identification results of the bacteria. Their similarity indexes in BIOLOG were changed from 45 to 76. None of them showed hypersensitivity positive reaction on tobacco plants.
The insecticidal activities of treatments tested against R. simulans nymphs and adults on tomato plants were given (Figs. 1, 2, 3, 4). According to these results, all the bacteria and fungi showed more or less insecticidal activity against pest in controlled conditions.
Table I.- MIS and BIOLOG identification results and HR (hypersensitivity) test results of the bacterial strains used in this study.
| Strain |
Pest (isolated from) |
MIS identification results |
SIM |
BIOLOG identification results |
SIM |
Reference |
|
CP-1 |
Ricania simulans |
Brevibacillus brevis |
0.65 |
Bacillus subtilis |
45 |
This study |
|
FDP-1 |
Malacosoma neustria |
Bacillus thuringiensis |
0.64 |
Bacillus thuringiensis |
56 |
This study |
|
FDP-8 |
Hypera postica | Bacillus thuringiensis subsp. kenyae |
0.45 |
Bacillus thuringiensis |
59 |
Tozlu et al. (2011) |
|
FDP-41 |
Apion spp. | Bacillus thuringiensis subsp. kurstakii |
0.57 |
Bacillus thuringiensis |
45 |
Tozlu et al. (2011) |
|
FDP-42 |
Apion spp. | Bacillus thuringiensis subsp. kenyae |
0.47 |
Bacillus thuringiensis |
75 |
Tozlu et al. (2011) |
|
FD-1 |
Malacosoma neustria |
Brevibacillus brevis |
0.65 |
Bacillus subtilis |
45 |
Tozlu et al. (2011) |
|
EK-7 |
Rosehip |
Bacillus subtilis |
0.65 |
Bacillus subtilis |
76 |
Tozlu et al. (2017) |
|
NEM-28 |
Ricania simulans |
Pseudomonas chlororaphis |
0.40 |
Pseudomonas sp. |
57 |
This study |
|
FD-49 |
Culex sp. |
Bacillus sphaericus GC subgroup D |
0.71 |
Bacillus sphaericus |
58 |
Dadasoglu (2013) |
|
BAB-410 |
Ricania simulans | Bacillus thuringiensis subsp. kurstakii |
0.62 |
Bacillus thuringiensis |
57 |
This study |
| X | Ricania simulans | Not done |
- |
Not done |
- |
This study |
SIM, similarity ındex; -, negative reaction; X, other tested bacterial strains and fungal isolates.
Their insecticidal effects changed 19.58 and 100 (Fig. 1) on nymphs. The highest mortality rate values were observed plant-origin pesticide containing the active substance Azadirachtin A and the pesticide containing the active substance Spinosad (100). FDP 42 (42.08), CP 1 (40.41), FD-49 (37.91), FD-1 (27.08), EK-7 (25.83), FDP-41 (25.83), NEM-28 (24.16), FDP-1 (21.25), ET 10 (entomopathogen fungi B. bassiana) (20), FDP-8 (20) and BAB-410 (19.58) followed these applications against nymphs. Six bacterial strains and 10 fungal isolates isolated from R. simulans nymphs and adults collected from the field were in the same group with the control. They were shown in Figure 1 as other bacteria and fungi tested. The lowest mortality rates were observed with control (0.41), other bacteria (0) and fungi tested (0) (Fig. 1).
The nymph mortality rates according to days during the 8 days follow-up period were given in Figure 2. All R. simulans nymphs died in Azadirachtin A and Spinosad applications on first day. The nymphs started to die in FDP 42, CP-1, FD 1, EK 7, FDP-41 and FDP-1 applications on second days and in FD-49, NEM-28, and ET 10 applications on thirds day (Fig. 2).
Insecticidal effects of bacterial and fungal isolates changed 11.33 and 100 (Fig. 3) on adults. The highest mortality rate values were observed Azadirachtin A and Spinosad (100) as applications against nymphs. FDP 1 (18.00), NEM-28 (17.33), FD-1 (17.33), BAB-410 (16.67), FDP-8 (16.67), FDP-42 (14.67), FD-49 (14.00), CP-1 (14.00), FDP-1 (13.33), EK-7 (11.33) AND ET 10 (6) followed these applications against adults. Other bacterial strains and other fungi tested in the same group with the control (Fig. 3). The lowest mortality rates were observed with control, other bacteria and fungi tested (0) (Fig. 3).
According to days during the 5 days follow-up period, the adult’s mortality rates were given in Figure 4. All R. simulans adults died in Azadirachtin A and Spinosad applications on first day. The nymphs started to die in FDP 42, CP-1, FD 1, EK 7, FDP-41 and FDP-1 applications on second days and in FD-49, NEM-28, and ET 10 applications on thirds day (Fig. 4).
Figure 4 showed mortality rates according to days during a follow-up period of 5 days. All adults were died in Azadirachtin A, Spinosad in first day. The adults started to die in FDP-41, NEM-28, FDP-8 and BAB-410 applications in first day, in FDP 42, CP-1, FD-49, FD 1, EK 7 and FDP-in second days and in ET10 applications in third days.
Discussion
There are many studies related to utilization of entomopathogenic bacteria and fungi in biological pest control (Inglis et al., 2001; Wraight et al., 2001; Lacey et al., 2001; Vestergaad et al., 2003; Copping, 2004; Aslantas et al., 2008; Khachatourians and Sohail, 2008; Ak et al., 2013, 2014). However, studies that investigate biological pesticides against the R. simulans are very limited.
One study that was conducted on tea leaves in laboratory and kiwi plant in field conditions tested 6 isolates of Lecanicillium muscarium (Petch) at a dose of 107 conidia/ml against nymphs of the pest, and found 50.95-74.76% mortality in 2.34-3.90 days, respectively; and a dose of 107 conidia/ml was effective against nymphs and adults in field conditions in 4.18-6.49 days, respectively. The authors concluded that L. muscarium could be an alternative and environment-friendly pest control agent against R. simulans (Guclu et al., 2010). Ak et al. (2014) applied Conidiobolus coronatus, another entomopathogenic fungus against this species, in both laboratory and field conditions, and they reported 100% success.
Sixteen bacterial strains from this species were isolated and 9 of them were identified as species and 7 of them were identified as genus in the other study. Among these, Pseudomonas sp. (Rs4 isolate) showed highest insecticidal activity at the nymph stage with 82% efficacy, while B. thuringiensis (Rs16 isolate) showed the highest insecticidal activity at the adult stage with 86% efficacy (Alev, 2014).
In this study, totally of 9 bacterial strains belonging to Bacillus genera, 1 bacterial strain belonging to Pseudomonas genera and 1 fungal isolates Beauveria bassiana were tested for insecticidal activities against R. simulans nymphs and adults. All of the tested bacterial strains and fungal isolate showed more or less insecticidal activity against the pest in controlled conditions. The most effective bacteria were B. thuringiensis FDP-42, B. subtilis CP-1 and B. sphaericus FDP 49 strains against nymphs and B. thuringiensis FDP-41, Pseudomonas sp. NEM-28 and B. subtilis FD-1 strains in adults. ET 10 against both nymphs and adults were different from control. Mortality rates were between 19.58-42.08% in nymphs, and between 6-18% in adults.
Studies conducted by different researchers have previously shown that Bacillus species can be successfully used in biological pest control (Gray et al., 2001; Alper et al., 2013), and particularly B. thuringiensis was reported to have approximately 2% share of the insecticide market (Bravo et al., 2007). B. bassiana, whose efficacy was tested in the present study, is one of the most important entomopathogens used in biological pest control (Zibaee et al., 2013). As an environment-friendly entomopathogen, the fungus B. bassiana has a wide host range and is commercially available (Keyhani, 2015). Although Al-Mazra’awi and Shipp (2006), reported that B. bassiana was given successful results in biological pest control against the Hemiptera species. In this study, we found that B. bassiana was more effective compared to control, while it showed less efficacy when compared to the studied bacterial strains.
Our results indicated that, the applications were more effective against nymphs than adults. These effects may be varied in field treatments. In the future, it is important that this study should be conducted in controlled conditions. We plan to prepare a commercial preparation after making a good carrier consisting of organic material with long shelf life for the most effective bacterial strain. In the midst of these obstacles, the bacterial strains B. thuringiensis, B. subtilis and B. sphaericus can be commercialized for management of R. simulans in both agricultural and horticultural crops.
ACKNOWLEDGEMENT
This study was supported by Department of Scientific Research Project Management of Atatürk University (BAP), with the project number 2014/105. We are thank to the Department of Scientific Research Project Management of Atatürk University (BAP)
Statement of conflict of interest
Authors have declared no conflict of interest.
References
Ak, K., Guclu, S. and Sekban, R., 2013. A new pest in East Black Sea Region, Ricania simulans (Walker, 1851) determining effectiveness of bio-pesticides with active substances of Azadirachtin and Spinosad against (Hemiptera: Ricaniidae). J. agric. Sci. Res., 6: 10-14.
Ak, K., Eken C., Guclu, S., Genc, T. and Sekban, R., 2014. Laboratory and field evaluation of the entomopathogenic fungus, Conidiobolus coronatus for controlling Ricania simulans (Walker, 1851) (Hemiptera: Ricaniidae). Egyptian J. biol. Pest Contr., 24: 455-459.
Ak, K., Guclu, S., Eken, C. and Sekban, R., 2015. New pest for Turkey: Ricania simulans (Walker, 1851) (Hemiptera: Ricaniidae). Türk. Ent. Derg., 39: 179-186. https://doi.org/10.16970/ted.94434
Alev, F., 2014. Determination of bacterial control agents of Ricania simulans. Blacksea Technical University, Science Institute, Graduate thesis, pp. 87.
Al-Mazra’awi, MS. and Shipp, L., 2006. Biological control of Lygus lineolaris (Hemiptera: Miridae) and Frankliniella occidentalis (Thysanoptera: Thripidae) by Bombus impatients (Hymenoptera: Apidae) vectored Beauveria bassiana in greenhouse sweet pepper. Biol. Contr., 37: 89-97. https://doi.org/10.1016/j.biocontrol.2005.11.014
Alper, M., Gunes, H., Civelek, H.S., Dursun, O. and Eskin, A., 2013. Toxic effects of some native Bacillus thuringiensis Berliner (Bacillales: Bacillaceae) isolates against Tetranychus urticae Koch (Acarina: Tetranychidae), Ceroplastes rusci L. (Homoptera: Coccidae) and Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). Turk. Ent. Bull., 3: 75-87.
Aslantas, R., Eken, C. and Hayat, R., 2008. Beauveria bassiana pathogenicity to the cherry slugworm, Caliroa cerasi (Hymenoptera: Tenthredinidae) larvae. World J. Microbiol. Biotechnol., 24: 119–122. https://doi.org/10.1007/s11274-007-9447-y
Bravo, A., Gill, S.S. and Soberon, M., 2007. Mode of action of Bacillus thuringiensis cry and Cyt toxins and their potential for insect control. Toxicon, 49: 423-435. https://doi.org/10.1016/j.toxicon.2006.11.022
Copping, L.G., 2004. The manual of biocontrol agents, 3rd ed. British Crop Protection Council, Alton, pp. 702.
Dadasoglu, F., Karagoz, K., Kotan, R., Sarihan, F., Yildirim, E., Sarac, S. and Haramtepe, F.B., 2013. Biolarvicidal effects of nine Bacillus strains against larvae of Culex pipiens Linnaeus, 1758 (Diptera: Culicidae) and non-target organisms. Egyptian J. Pest Contr., 23: 35-42.
Deshpande, M.V., 1999. Mycopesticide production by fermentation: Potential and challenges. Crit. Rev. Microbiol., 25: 229-243. https://doi.org/10.1080/10408419991299220
Gokturk, T. and Aksu, Y., 2014. Morphology, biology and damage of Ricania simulans (Walker 1851) (Hemiptera: Ricaniidae) which damages in the agriculture and forest areas. Symposium of Turkey Forest Entomology and Pathology 7-9 April, Antalya, Turkey, pp. 282-285.
Gray, L.R., Jensen, A., Riebe, J., Head, G. and Duan, J.J., 2001. Transgenic Bacillus thtungiensis potato and conventional insecticides for Colorado potato beetle management: comparative efficacy and non-target impacts. Ent. Exp. Appl., 100: 89-100. https://doi.org/10.1046/j.1570-7458.2001.00851.x
Guclu, S., Ak, K., Eken, C., Akyol, H., Sekban, R., Beytut, B. and Yildirim, R., 2010. Pathogenicity of Lecanicillium muscarium against Ricania simulans. Bull. Insectol., 63: 243-246.
Holmes B., Costas, M., Ganner, M., On, S.L. and Stevens, M., 1994. Evaluation of Biolog system for identification of some gram negative bacteria of clinical importance. J. clin. Microbiol., 32: 1970-1975.
Huang, Z., Ali, S., Ren, S., Wu, J. and Zhang, Y., 2012. Influence of entomopathogenic fungus Beauveria bassiana on Prynocaria congener (Billberg) (Coleoptera: Coccinellidae) under laboratory conditions. Pakistan J. Zool., 44: 209-216.
Inglis, G.D., Goettel, M.S., Butt, T.M. and Strasser, H., 2001. Use of hyphomycetous fungi for managing insect pests. In: Fungi as biocontrol agents: Progress, problems and potential (eds. T.M. Butt, C. Jackson and N. Magan). CABI Publishing, pp. 23–69. https://doi.org/10.1079/9780851993560.0023
Keyhani, N.O., 2015. A beetle versus a fungus: Tribolium castaneum, a pest of stored grains and insect pathogenic fungus Beauveria bassiana (invited paper). 5th Entomopathogens and Microbial Control Congress, 9-11 September 2015. Ankara University, Ankara, Turkey.
Khachatourians, G.G. and Sohail, S.Q., 2008. Entomopathogenic fungi. In: Biochemistry and molecular biology, human and animal relationships (eds. A.A. Brakhage and P.F. Zipfel), 2nd Edition. The Mycota VI, Springer-Verlag, Berlin, Heidelberg.
Klement, Z., Rudolph, K. and Sands, D., 1964. Hypersensitive reaction induced by phytopathogenic bacteria in the tobacco leaf. Meth. Phytobacteriol. Phytopathol., 54: 474-477.
Lacey, L.A., Frutos, R., Kaya, H.K. and Vail, P., 2001. Insect pathogens as biological control agents: Do they have a future? Biol. Contr., 21: 230–248. https://doi.org/10.1006/bcon.2001.0938
Quesada-Moraga E., Maranhao E.A.A., Valverde- Garc L.P. and Santiago-Allvarez C., 2006. Selection of Beauveria bassiana isolates for control of the whiteflies Bemisia tabaci and Trialeurodes vaporariorum on the basis of their virulence, thermal requirements and toxicogenic activity. Biol. Contr., 36: 274-287. https://doi.org/10.1016/j.biocontrol.2005.09.022
Sasser, M., 1990. Identification of bacteria through fatty acid analysis. In: Methods in phytobacteriology (eds. Z. Klement, K. Rudolph and D. Sands). Academia Kiado, Budapest, pp. 199-204.
Sezen, K. and Demirbag, Z., 2007. Bacterial isolates from Palomena prasina (Hemiptera: Melolontha, Coleoptera : Scarabaeidae). Ecology, 16: 34-40.
Thiery, I. and Frachon, E., 1997. Bacteria: identification, isolation, culture and preservation of entomopathogenic bacteria. In: Manual of techniques in insect pathology (ed. L.A. Lacey), Cap. III-1. Biological Techniques Series, Academic Press, London, pp. 55-75. https://doi.org/10.1016/B978-012432555-5/50006-3
Tozlu, E., Dadasoglu, F., Kotan, R. and Tozlu, G., 2011. Insecticidal effect of some bacterıa on Bruchus dentipes Baudi (Coleoptera: Bruchidae). Fresenius environ. Bull., 20: 918-923.
Tozlu, E., Kotan, R. and Tozlu, G., 2017. The investigation of Beauveria bassiana (Ascomycota: Hypocreales) as a biocontrol agent of rose-stem sawfly, Syrista parreyssii (Spinola, 1843) (Hymenoptera: Symphyta; Cephidae) larvae. Fresenius environ. Bull., Under process.
Tozlu, E., Mohammadi, P., Senol Kotan, M., Nadaroglu, H. and Kotan, R., 2016. Biological control of Sclerotinia sclerotiorum (Lib.) de Bary, the causal agent of white mould disease in red cabbage, by some bacteria. Pl. Protect. Sci., 52: 188-198. https://doi.org/10.17221/96/2015-PPS
Vestergaard, S., Cherry, A., Keller, S. and Goettel, M.S., 2003. Safety of hyphomycete fungi as microbial control agents. In: Environmental impacts of microbial insecticides (eds. H.M.T. Hokkanen and A.E. Hajek). Kluwer Academic Publishers, Dordrecht, pp. 35-62. https://doi.org/10.1007/978-94-017-1441-9_3
Wraight, S.P., Jackson, M.A. and de Kock, S.L., 2001. Production, stabilization and formulation of fungal biocontrol agents. In: Fungi as biocontrol agents: Progress problems and potential (eds. T.M. Butt, C. Jackson and N. Magan). CABI Publishing, pp. 253–287. https://doi.org/10.1079/9780851993560.0253
Zibaee, A., Bandani, A.R. and Sendi, J., 2013. Pathogenicity of Beauveria bassiana to fall webworm (Hyphantria cunea) (Lepidoptera: Arctiidae) on different host plants. Pl. Protect. Sci., 49: 169–176.
To share on other social networks, click on any share button. What are these?