Studies on the Recombinant Production in E. coli and Characterization of Pharmaceutically Important Thermostable L-Asparaginase from Geobacillus thermodenitrificans
Studies on the Recombinant Production in E. coli and Characterization of Pharmaceutically Important Thermostable L-Asparaginase from Geobacillus thermodenitrificans
Muhammad Shahid Nadeem*, Maryam A. Al-Ghamdi and Jalaluddin Azam Khan
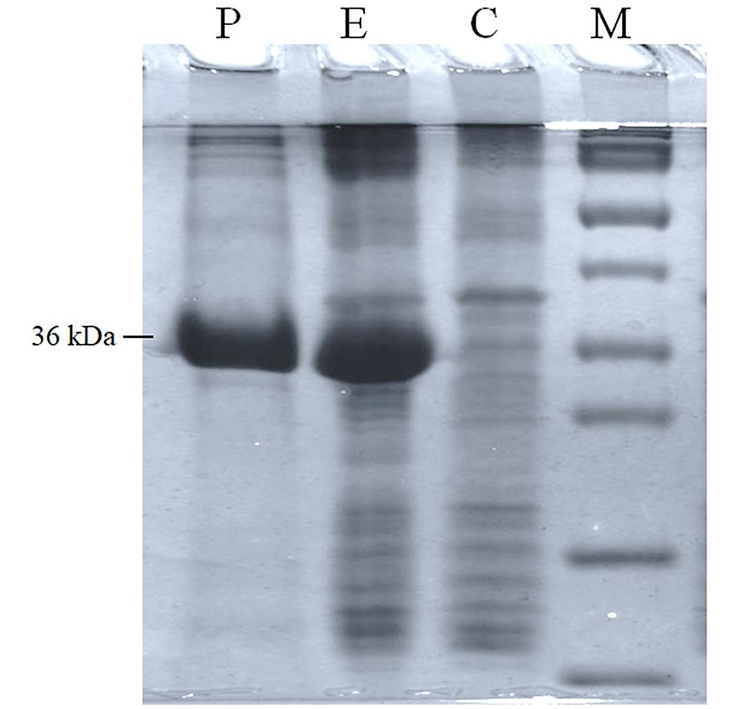
SDS-PAGE image showing partially purified enzyme (Lane P), gene expressed in E. coli (Lane E), negative control (Lane C) and protein marker (Lane M).
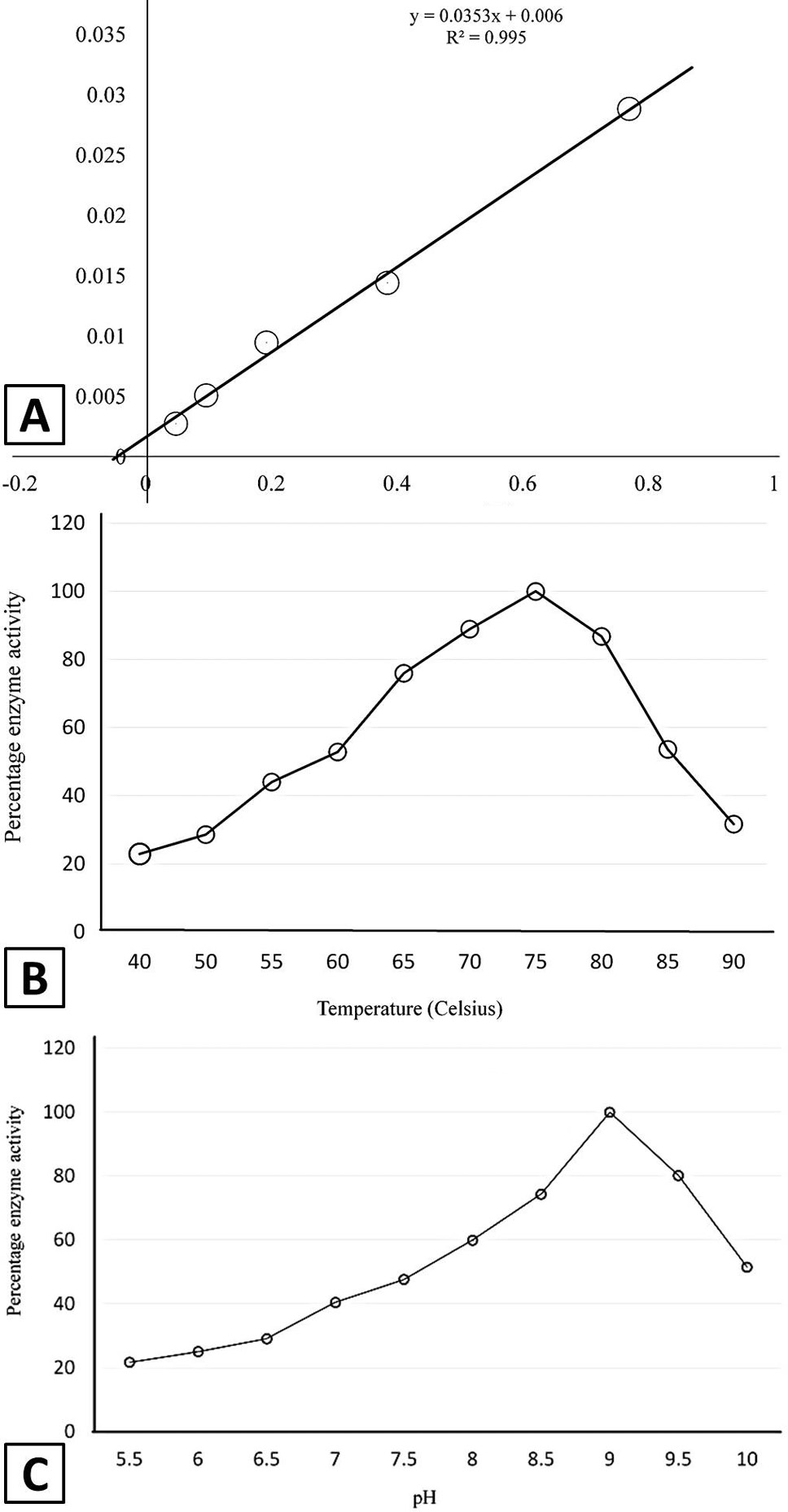
Kinetic properties of recombinant asparginase. A, effect of substrate concentration on the enzyme activity indicating KM value of 5.9mM for L-Asparagine; B, effect of temperature on the enzyme activity; C, effect of pH, on the activity of enzyme.
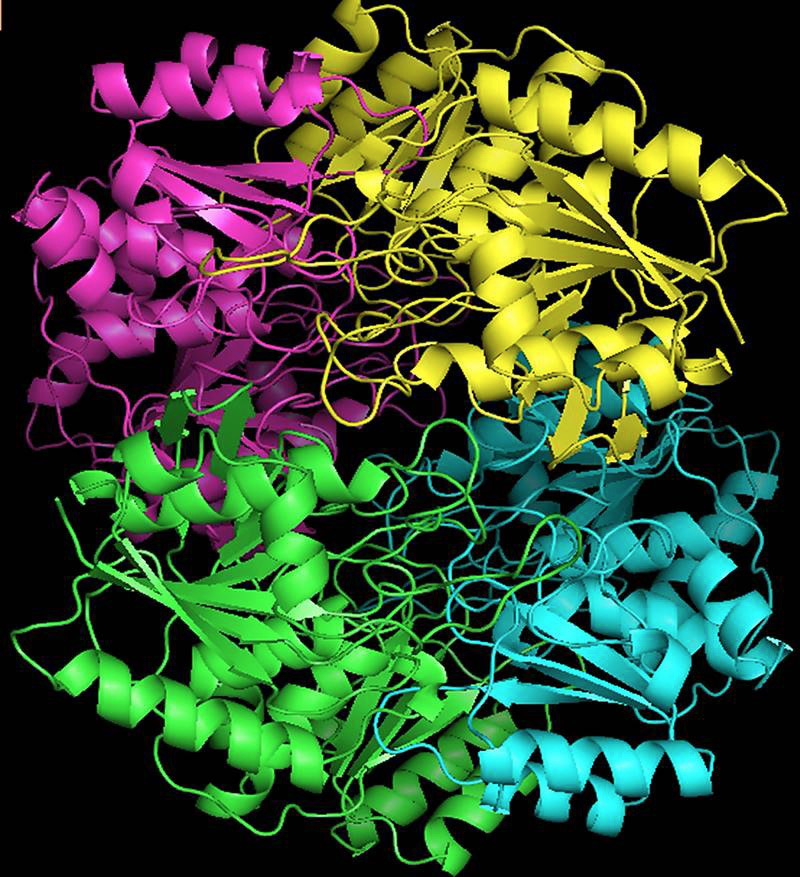
ASP-GT monomer built by SWISS server, depicted in rainbow cartoons highlighting the secondary structure with different colours.
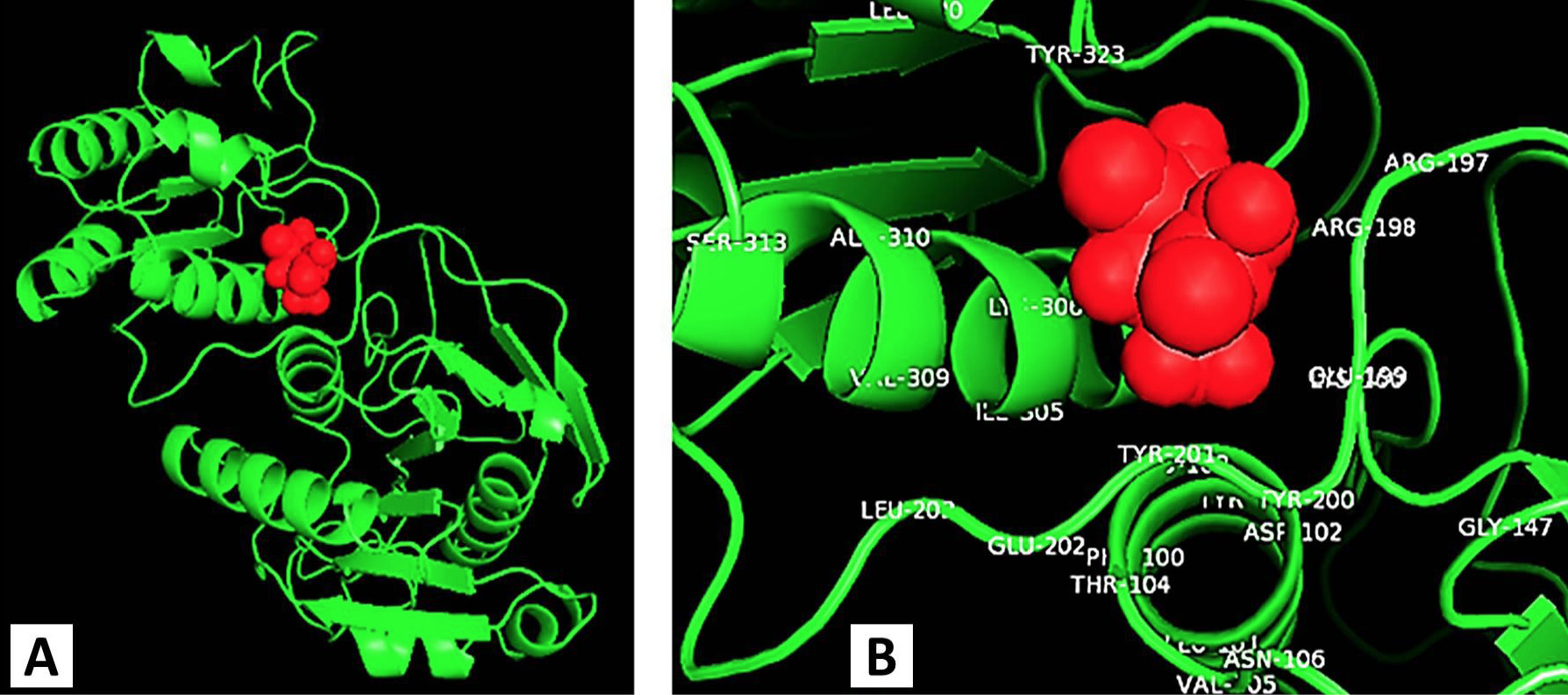
Molecular docking photograph indicating the binding site residues of L-asparaginase with its substrate (L-asparagine).