Synthesis, Characterization and Investigation of the Anticoccidial Activity of New formulation Curcumin - Olive Oil Nano-Composite
Research Article
Synthesis, Characterization and Investigation of the Anticoccidial Activity of New formulation Curcumin - Olive Oil Nano-Composite
Reem M. Ramadan1, Fady Sayed Youssef2, Gehad Genidy Mohamed3, Sameh Hamed Ismail4, M.M. El-Bahy1, Shimaa Abdel-Radi1*
1Department of Parasitology, Faculty of Veterinary Medicine, Cairo University, 1221 Giza, Egypt; 2Department of Pharmacology, Faculty of Veterinary Medicine, Cairo University, 1221 Giza, Egypt; 3Chemistry Department, Faculty of Science, Cairo University, 12613 Giza, Egypt and Nanoscience Department, Basic and Applied Sciences Institute, Egypt-Japan University of Science and Technology, New Borg El Arab, Alexandria, 21934, Egypt; 4Faculty of Nanotechnology for postgraduate studies - Cairo University- Sheikh Zayed Branch Campus, Sheikh Zayed City, Giza PO 12588, Egypt.
Abstract | New formulation of nanocurcumin (curcumin-olive oil nano-composite;
C-Oo.Nc) was produced and characterized in the present study. The produced nanoparticles are spherical with 63 nm zeta size and high stability in the aqueous media. The coccidiocidal efficacy of the C-Oo.Nc was evaluated versus five chicken Eimeria species oocysts. The immature oocysts were exposed to 5, 10, 20, and 40 ppm, each for 1, 3, 6, 9, 12, 24, and 36 h. Percentage of sporulation inhibition in oocysts and comet assay were used to investigate the C-Oo.Nc destructive effects. The infectivity of sporulated oocysts exposed to LC50 & LC90 were evaluated by In vivo inoculation of broiler chicks. The results demonstrated that the increase in the rate of sporulation inhibition is directly related to the increase in C-Oo.Nc concentrations and exposure time. Analyzing the DNA damage in the oocysts exposed to different concentrations using comet assay revealed a significant direct variation (p ≤ .05) between the increase in the dose and the degree of the DNA genotoxic damage as represented by variations in the tail length (μm), percent of DNA in the tail segment, and tail moment (μm). Postmortem (PM) inspection of birds inoculated by LC90 exposed sporulated oocysts demonstrated a very slight or un-apparent reddens without thickening in the cecal wall with close relation to the normal un-infected control group. This with in contrast to the group inoculated by LC50 exposed sporulated oocysts. The study concluded that C-Oo.Nc is a promising effective anticoccidial compound for controlling infection in chickens.
Keywords | Curcumin -olive oil nanocomposite, anticoccidial activity, Comet assay, Poultry, coccidiosis.
Received | July 25, 2022; Accepted | August 15, 2022; Published | September 20, 2022
*Correspondence | Shimaa Abdel-Radi, Department of Parasitology, Faculty of Veterinary Medicine, Cairo University, 1221 Giza, Egypt; Email: shimaa.abdelradi@cu.edu.eg
Citation | Ramadan RM, Youssef FS, Mohamed GG, Ismail SH, El-Bahy MM, Abdel-Radi S (2022). Synthesis, characterization and investigation of the anticoccidial activity of new formulation curcumin - olive oil nano-composite. Adv. Anim. Vet. Sci. 10(10): 2186-2196.
DOI | http://dx.doi.org/10.17582/journal.aavs/2022/10.10.2186.2196
ISSN (Online) | 2307-8316

Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Nanotechnology has been considered as a promising field for generating innovative materials with dimensions ranging from 1 to 100 nanometers since 1974. In the metric system, “Nano” equals a billionth, so a nanometer is one-billionth of a meter (units of 10 -9 meters) (Elgadir et al., 2015). Nanotechnology has significant potential in the field of medicine including veterinary medicine as it is enhancing drug or other medicinal products delivery with special significance for biomedical applications in vivo and In vitro (Youssef et al., 2021).
Coccidiosis is a serious parasitic disease in the poultry industry which is one of the main suppliers of animal protein worldwide. It is caused by the genus Eimeria which belongs to the phylum Apicomplexa. Seven Eimeria species were identified in poultry, each targeting a specific part within the intestines with different pathogenicity (Pop et al., 2019; Mesa-Pineda et al., 2021). Poultry coccidiosis causes high morbidity, low growth rate, increased susceptibility to other diseases, and high mortalities during severe infection leading to high economic losses in poultry farms (Santos et al., 2020).
Different anti-coccidial drugs have been used to prevent and control coccidiosis. However, the excessive usage of these drugs may cause drug resistance and residues in tissues (Yadav and Jha, 2019). To overcome this issue; new drugs, drugs combinations, and different methods of administration such as supplements with antioxidants (Abdel-Rahman and Abdel-Radi, 2022) or testing other related drugs (Abou-Okada et al., 2021) were applied, but this increases the cost of the poultry industry (Jamil et al., 2022). Moreover, the rotational program of vaccines and drugs is a common strategy (Hafez, 2008). In addition, supplying ionophores and some feed additives have been used to reduce coccidiosis in poultry (Teng et al., 2020). Due to the side effects of anti-coccidial drugs, the disadvantages of conventional vaccines, ionophores poultry toxicity, and resistance of some Eimeria spp. to some ionophores and anti-coccidial drugs (Wunderlich et al., 2014), the research directed towards using some new natural alternatives and exploring the antimicrobial activity of different herbal plants or their extracts which are specific, effective, inexpensive and safe to consumers in order to limit the emergence of resistance in Eimeria strains and to extend the effectiveness of available anticoccidial medications (Lillehoj et al., 2018; Pop et al., 2019; Raza et al., 2022).
One of the studied natural compounds in this respect is curcumin, a polyphenolic compound extracted from the Curcuma longa herbal plant, commonly known as turmeric (Kocaadam and Sanlier, 2017). Turmeric is an ancient perennial herb native to India that belongs to the Zingiberaceae family and is widely farmed in tropical and subtropical regions of the world (Esatbeyoglu et al., 2012).
Curcumin has different pharmacological effects such as anti-inflammatory, anti-mutagenic, antitumoral, wound healing, and anti-angiogenesis properties. Also, it has gastroprotective, antiproliferative, antiarthritic, and neuroprotective effects for humans and animals (Willenbacher et al., 2019). Moreover, it has an antioxidant and antimicrobial property, effectively reducing poultry coccidia and other protozoa infections (Abbas et al., 2010; Nagajyothi et al., 2012). Many studies were performed to investigate the anti-coccidial activity of curcumin on different Eimeria spp. Curcumin reduces the small intestinal infections caused by E. acervulina and E. maxima. Furthermore, curcumin can destroy sporozoites of E. tenella (Khalafalla et al., 2011), affect the wall of the oocysts, and reduce the oocyst shedding and gut lesions (Fatemi et al., 2015). Moreover, it can inhibit the sporulation of the exposed oocysts (Felici et al., 2021).
The normal form of curcumin is highly lipophilic and insoluble in water at basic pH. Moreover, it is unstable in working solution and degrades during long timed sample preparation. These lead to its incomplete absorption, poor bioavailability, and low organ penetration property (Ajay et al., 2012). Tsai et al. (2011) reported that the nano form of curcumin is easily dissolvable in water and has a significantly higher penetration and distribution rate. Therefore, the researchers directed to minimize the average diameter of curcumin solid particles and use the nano-curcumin in controlling coccidiosis (Gogoi et al., 2019). Using nanocurcumin as a dietary supplementation at low doses is safe and effective on carcass characteristics and physical and chemical properties of broiler chicken meat infected with Eimeria acervulina, Eimeria maxima, and Eimeria tenella (Partovi et al., 2019).
In the present study, curcumin-olive oil nano-composite (C-Oo.Nc) was originally prepared and characterized. Its coccidiocidal efficacy was evaluated against five Eimeria species of poultry. Moreover, its sporulation inhibitory effects were evaluated on immature oocysts. The effect of its LC50 & LC90 on the infectivity of sporulated oocysts was evaluated by inoculation to broiler chicken. Finally, the degree of DNA genotoxic damage in the exposed and control oocysts was analyzed using the comet assay.
MATERIALS AND METHODS
The tested curcumin-olive oil nano-composite (C-Oo.Nc)
Materials: Curcumin was purchased from (Sigma Aldrich Company) and olive oil was bought from (Sekem Company, Cairo, Egypt). While Tween 80 was purchased from (Al Nasr Company for Pharmaceutical Chemicals, Cairo, Egypt). All substances were used in a pure form without any modification.
Curcumin nanoparticles were synthesized by sonochemical methods. Curcumin was exposed to ultrasonic waves using a specialized sonicator (Sonorex Digitec, Model: Andelin; 35 kHz, Germany) which led to the formation and growth of micro-bubbles. The formed micro-bubbles have an extreme temperature (45ºc) and pressure (P 20 Mega Pascal) on the inner and outer sides of bubbles. When collapsing, the curcumin molecules subjected to these extreme conditions cause nucleation of nanoparticles, then rapid cooling lead to the synthesis of curcumin nanoparticles.
For the preparation of nano-curcumin oil-in-water (O/W), the oil phase and surfactant (Tween 80) were mixed (by a ratio of 1:9 w/w) under continuously stirring for 15 minutes to form the oily phase. The oil acts as a stabilizer, coating curcumin nanoparticles and preventing aggregation. However, 0.01 g of curcumin was added to a 10 ml oil phase and then let under a magnetic stirrer for stirring for 1 h at 600 rpm. Then the solution was subjected to sonication, and the mixture was placed in a sonicator bath for 1 hour to obtain a clear yellow homogeneous oily solution. Subsequently, the final nanocomposite was formed by adding deionized water to the oily phase (5:1 w/w) and preceded with stirring at 500 rpm for 30 minutes under a temperature not exceeding 80oC. The total sonication time is 1.5 hours with rest intervals of 1 second, under 75% amplitude. Finally, the materials were supplied as a solution; each 1.0 ml contains 100 ppm of the active material. The product was easily dissolved in water to reach the required concentrations.
Characterization of the prepared (C-Oo.Nc): Characterization is classified into two sectors; identification and shape-size sector. The identification sector was carried out to confirm the synthesis without any contamination or change in the chemical or crystallography of curcumin nanoparticles. However, the shape-size sector was performed to illustrate the morphology of curcumin nanoparticles. Shape-size sector is classified into two subsectors; the size sector carried out by DLS using Malvern Instruments Ltd. Model of Nano Sight NS500 and the shape sector demonstrated by SEM (Prisma E Thermo Scientific Company) and TEM (EM-2100 High-Resolution at magnification).
C-Oo.Nc was produced in the Departement of Pharmacology, Faculty of Veterinary Medicine, Cairo University, Egypt. While it is characterized in the Nano-science Department, Basic and Applied Sciences Institute, Egypt-Japan University of Science and Technology, New Borg El Arab, Alexandria, 21934, Egypt and in Faculty of Nanotechnology for postgraduate studies - Cairo University Sheikh Zayed Branch, Giza PO 12588, Egypt.
Reference drug
As a standard anti-coccidial medication, Amprolium (20 percent amproxine, Water Soluble Powder, Pharma Swede Pharmaceuticals Co.Ltd., Egypt) was used. They were given their concentration and exposure time in accordance with the manufacturer’s recommendations. The medication was used at 240 ppm (El-Khtam et al., 2014; Felici et al., 2021).
The used Eimeria species oocysts
Eimeria spp. oocysts were obtained from naturally infected ceca collected from freshly slaughtered natural infected chickens at the Department of Parasitology, Faculty of Veterinary Medicine Cairo University; according to (Chand et al., 2016). The caecal contents were collected, homogenized then sieved to remove the coarse fecal debris, and the oocysts were cleaned by washing several times and precipitation in water. The oocysts were separated by concentration flotation technique, then identified according to (El-Khtam et al., 2014), and then kept in potassium dichromate (2.5%) in a refrigerator until used. Five Eimeria spp. oocysts were detected and used in the present study in the following percentage; E. tenella 65%, E. maxima 12%, E. acervulina 10%, E. neatcrix 8%, and E. mitis 5%.
Testing and evaluating the coccidiocidal effects of C-Oo.Nc
Anti-coccidial effects of the produced (C-Oo.Nc) were tested versus Eimeria spp. un-sporulated and sporulated oocysts. The cleaned, freshly collected Eimeria spp. un-sporulated oocysts were spread as a thin layer in the bottom of 6 cm diameter Petri-dishes for a series of upgraded concentrations of C-Oo.Nc; 5, 10, 20, and 40 ppm each for 1, 3, 6, 9, 12, 24, and 36 hours of exposure time in duplicates at room temperature. Amprolium (20%) was used simultaneously at a concentration of 240 ppm as a reference drug (Felici et al., 2021); moreover, control oocysts in distilled water were associated with also. A tested solution was removed at the end of the exposure time, and the oocysts were cleaned by washing and sedimentation several times. The exposed immature oocysts were incubated at 28ºC for 5-7 days in potassium dichromate for sporulation.
To evaluate the coccidiocidal effect of C-Oo.Nc on the exposed Eimeria spp. un-sporulated oocysts, sporulated and non-sporulated oocysts were counted using the McMaster technique (Chand et al., 2016), then the percentage of sporulation inhibition was calculated by counting the number of unsporulated oocysts from a total of 100 oocysts (3 replicates) for each concentration. The sporulation inhibition percentage was calculated using the following equation;
Sporulation inhibition %
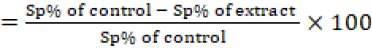
In addition, single-cell gel electrophoresis (SCGE) / comet assay was adopted to evaluate the effect of the products on the DNA damage in the exposed oocysts (Singh et al., 1988).
In order to evaluate the coccidiocidal effect of C-Oo.Nc on Eimeria spp. sporulated oocysts, a sufficient number of the collected immature oocysts from the naturally infected chickens, were sporulated by keeping them in 2.5% potassium dichromate solution in Petri-dishes, then incubated at 28ºC for 5-7 days. Potassium dichromate was washed off, then the adjusted number of sporulated oocysts counted by the McMaster technique was exposed to the calculated LC50%, and LC90% of (C-Oo.Nc) previously recorded after exposure of the immature oocysts. After that, the comet assay was performed to evaluate the effect of the products on the DNA damage in the exposed oocysts (Singh et al., 1988). Moreover, the destructive effect of C-Oo.Nc was evaluated on the sporulated oocysts exposed to the calculated LC50 & LC90 (Salama et al., 2012) by In vivo inoculation to chicks as the following:
Chicken: One hundred of one-day-old Hubbard broiler chicks were obtained from a local broiler hatchery. They were kept under observation till became of 14-days-old chicks. All chicks were kept under good hygienic measures supplied by water and a balanced ration free from anti-coccidial drugs. Before inoculations, their feces were microscopically examined to ensure they were free from infection.
In vivo inoculation: The 14-days-old chicks were kept in separate nine equal groups, each of ten chicks. The groups were divided as the following:
Table 1: Evaluation of the infectivity of the exposed sporulated oocysts (Experimental design).
| Chicken groups (n=10) |
In vivo study |
|
| Control groups | G1 | Non-inoculated chickens (control negative) |
| G2 | Inoculated chickens with non-exposed sporulated oocysts (control positive) | |
|
Inoculated with sporulated oocysts exposed to the calculated LC50 |
G3 | 5.0 ppm C-Oo.NC |
| G4 | 10.0 ppm C-Oo.NC | |
| G5 | 20.0 ppm C-Oo.NC | |
| G6 | 40.0 ppm C-Oo.NC | |
|
Inoculated with sporulated oocysts exposed to the calculated LC90 |
G7 | 20.0 ppm C-Oo.NC |
| G8 | 40.0 ppm C-Oo.NC | |
| Reference drug group | G9 | sporulated oocysts exposed to amprolium 20% in a dose of 240 ppm |
The oocysts were orally inoculated/bird (approximate dose of 7 X 103) (El-banna et al., 2005). The chickens were kept under observation. Clinical symptoms and the presence of bloody feces were observed. Moreover, calculating the mean total number of shed oocyst/grams of feces/bird per each group during the whole period (5th to 10th dpi) was determined. In addition, slaughtering of the birds, post-mortem (PM) inspection, and the lesion score were determined in each case at the end of the experiment (12th dpi).
PM scoring of inoculated birds: PM inspection was performed for the whole intestine of birds inoculated by sporulated oocysts previously exposed to LC50 & LC90 and control non-exposed oocysts. The lesion score was determined according to El-Banna et al. (2005) at the end of the experiment as the following:
Grade 0 (-): There is no lesion recorded.
Grade 1 (+): There is a slight degree of redness with mild thickening associated with 1 to 3 focal lesions in a 3 cm2 of the intestinal wall.
Grade 2(++): The intestinal wall has moderate redness and thickening with focal lesions (3-6) in a 3 cm2 of the wall with ballooning in the caecum.
Grade 3 (+++): The intestine wall has severe congestion with more thickening associated with ballooning of the caecum and the presence of a “bloody cecal core” of variable degrees.
Evaluation of DNA damage by the comet assay
The comet assay was used to analyze the DNA damage as described by Singh et al. (1988). Visualization of DNA damage in the exposed and controlled Eimeria spp. oocysts were visualized in ethidium bromide-stained DNA using a fluorescent microscope (40x objective) with Komet 5 image analysis software (Kinetic Imaging, Ltd. Liverpool, UK), attached by a CCD camera that can assess the quantitative and qualitative extent of DNA damage in the cells by measuring the length and the percentage of DNA migration. Then the program calculates the tail moment. The damage to DNA was measured by the percentage of DNA in the tail (% DNA tail). One hundred cells per treatment were selected randomly and analyzed. The score of the comet assay was calculated according to the formula of Singh et al. (1988) in the range of 0- 400 arbitrary units.
Statistical analysis
The obtained data were statistically analyzed using analysis of variance (ANOVA), and comparing groups were performed using the least significant difference (LSD) at p ≤ 0.05 according to Petrie and Watson (1999) and computerized using SPSS II (2001).
RESULTS
Characterization of the prepared (C-Oo.Nc)
Scanning (SEM) and transmission (TEM) electron microscope characterization: SEM 3D and TEM 2D images for curcumin-olive oil nano-composite (C-Oo.Nc), as illustrated in (Figure 1), confirmed the nano-composite formation. They illustrated the spherical structure of curcumin nanoparticles in nanosized form.
Table 2: Mean % of sporulation inhibition in the exposed immature Eimeria spp. oocysts.
| Tested conc. |
Mean ±SD of inhibition of sporulation in Eimeria spp. oocysts after exposure to |
||||||
| 1h | 3h | 6h | 9h | 12h | 24h | 36h | |
|
C-Oo.NC 5.0 ppm |
7.8±1.3c |
27.6 ± 1.14c |
36.4 ±0.87c |
46.6 ±1.4c |
58.8 ± 0.85c |
70.8 ± 0.8b |
77.2 ± 1.5b |
|
C-Oo.NC 10.0 ppm |
27.6±1.14b |
36.6 ± 0.83b |
53.6 ±1.14b |
63.2 ±2.86b |
67.4 ± 1.4 bc |
78.2 ±0.9b |
85.4 ± 1.3ab |
|
C-Oo.NC 20.0 ppm |
36.2± 0.84ab |
40.8 ± 0.84b |
65.2 ±0.8ab |
69.2±1.79ab |
71.8 ± 1.1bc |
86.3±0.84ab |
95.8 ±2.1a |
|
C-Oo.NC 40.0 ppm |
42±0.7a |
65.4 ±0.89a |
75.6 ±1.1a |
81.6 ±1.8a |
87.6±1.13b |
94.8 ±1.3a |
98.7 ±0.9a |
|
Amprolium 20% 240 ppm |
34.5± 0.17ab |
41.3± 0.12b |
56.5± 0.11b |
76.3±0.8b |
98.7 ±0.9a |
98.7 ±0.9a |
- |
| Control -Ve | 0.00 | ||||||
* Concentration before these concentrations did not induce any mortalities.
* Data represented as mean ± SD.
*a-c Means with different superscripts within the same column are significantly (p-value ≤ 0.05) different.
Atomic force microscope (AFM) study: AFM analysis was performed for the C-Oo.Nc to confirm the shape, size, concentration, and agglomeration obtained from TEM and SEM data. AFM images (Figure 2) illustrated the spherical structure of curcumin-olive oil nanocomposite with a maximum thickness of less than 50 nm. It was clear from the figure that the C-Oo.Nc does not tend to form agglomeration in certain areas.
Zeta size and potential: Zeta size and potential were measured for curcumin nanoparticles to determine their size and stability in aqueous media. The curcumin nanoparticles size was 63 nm with a zeta potential of -24 mV. The size obtained matched the size given by TEM, SEM, and AFM images, and zeta potential values illustrated the good stability of the C-Oo.Nc in the aqueous media.
Coccidiocidal effect of the C-Oo.Nc
To study the coccidiocidal effect of the C-Oo.Nc, the sporulated immature Eimeria spp. oocysts were exposed to 5, 10, 20, and 40 ppm of the C-Oo.Nc each for 1, 3, 6, 9, 12, 24, and 36 hours. The data illustrated in (Table 2 and Figure 3) revealed that the increase in the rate of sporulation inhibition is directly related to the increase in C-Oo.Nc concentrations and exposure time. Exposing the oocysts to 5 ppm induced only 7.8 ± 1.3 % mortalities in the oocysts exposed for 1 h. This increased gradually to 77.2 ± 1.5 % after 36 h of exposure. Ten ppm revealed 27.6 ± 1.14% mortalities increased to 85.4 ± 1.3% after 36 h of exposure. At the same time, 20 ppm revealed 36.2 ± 0.84 % mortalities increased to 95.8 ± 2.1 % after 36 h, while the maximum mortalities were recorded after exposure to 40 ppm as it reached 42 ± 0.7% after 1 h exposure time and increased to 95.8 ± 2.1% then to 98.7 ± 0.9% after 48 and 36 hours, respectively. On the other hand, amprolium 20% at a concentration of 240 ppm revealed inhibition reached 98.7 ± 0.9% after 12 h exposure time with neglected sporulation inhibition of the control during calculation in each time.
The data in (Table 3 and Figure 3) demonstrated the calculated LC50 & LC90 for each tested concentration. The LC50 was 5 ppm/9 h, 10 ppm/5 h, 20 ppm/4 h, and 40 ppm/90 minutes exposure time. The 20 ppm and 40 ppm concentrations only proved that they could inhibit sporulation of the exposed immature oocysts after 28 and 15 hours of exposure, respectively.
Table 3: The calculated LC 50 LC90 of C-Oo.Nc versus Eimeria spp. unsporulated oocysts.
| LC50 |
LC90 |
| 5.0 ppm/ 9 h | -- |
| 10.0 ppm/ 5 h | -- |
| 20.0 ppm/ 4h | 20.0 ppm/ 28 h |
| 40.0 ppm / 1.30 h | 40.0 ppm / 15 h |
Effect of the drug LC50 and LC90 on the infectivity of the sporulated oocysts
Different groups of chicks were inoculated by sporulated oocysts exposed to the calculated LC50 & LC90 of the C-Oo.Nc and amprolium 240 ppm/24 h and then counted for the total number of oocysts/gram feces/bird that shed during the 5th-10th dpi. The mean total number of oocysts shed per gram of feces/bird revealed a marked decrease in the mean number of oocysts shed from the group exposed to LC90 (1633 ± 153 to 1500 ± 265) and amprolium 240 ppm/24 h (1432 ± 153) as well as a significant decrease (p ≤ 0.05) in the number of shed oocysts in groups exposed to LC50 (11473 ± 851 to 10100 ± 500) in comparison with (32633 ± 850) shed from control birds inculcated by the same number of unexposed oocysts (Table 4).
PM inspection of these birds at the 12th dpi revealed a marked difference (Figure 4). Only very slight or un-apparent redness without thickening in the wall was closely related to the normal un-infected control in the group inoculated by oocysts exposed to LC90. In contrast, those inoculated by oocysts exposed to LC50 revealed scores between grades 1 and 2 (+ and ++ ) (Figure 4), as their intestinal wall have light to moderate redness with mild thickening associated with sporadic focal lesions without ballooning of the ceca. At the same time, severe infection regarding grade 3 (+++) was recorded in all birds inoculated by the non-exposed oocysts (Figure 4). The intestine wall has severe congestion with more thickening associated with ballooning of the caecum and presence of “bloody cecal core” of variable degrees.
Table 4: Mean number of shed oocysts/birds inoculated groups during 5th - 10th dpi.
|
Dose of C-Oo.NC |
Mean number of shed oocyst/bird/gram feces during the whole period of oocysts exposed to |
Grad of Lesion Score
|
|
| LC 50 | 5.0 ppm/ 9 h |
11473± 851b |
Grade 2 (++) |
| 10.0 ppm/ 5 h |
11460±943b |
Grade 2 (++) | |
| 20.0 ppm/ 4h |
10770±580b |
Grade 1 (+) | |
| 40.0 ppm / 1.30 h |
10100±500b |
Grade 1 (+) | |
| LC 90 | 20.0 ppm/ 28h |
1633±153c |
Grade 0 (-) |
| 40.0 ppm / 15 h |
1500±265c |
Grade 0 (-) | |
|
Amprolium (20%) 240 ppm/24h |
1432 ±153c |
Grade 0 (-) | |
| Control non-exposed oocysts |
32633 ±850a |
Grade 3 (+++) | |
* Data represented as mean ± SD.
*a-c Means with different superscripts within the same column are significantly (p-value ≤ 0.05) different.
DNA damage in exposed oocysts (Comet assay)
The comet assay was used to analyze the DNA damage in the exposed and control Eimeria species oocysts. DNA damages were visualized in immature oocysts exposed to the tested four concentrations of C-Oo.NC after 24 h exposure time, for sporulated oocysts exposed to LC90 (20 ppm/28 h and 40 ppm/15 h) as well as in control (ampro
Table 5: Comet parameters and level of damage in DNA of Eimeria spp oocysts after 24 h exposure to different concentrations of nano-curcumin.
|
Tested concentration
|
% of sporulation inhibition |
% of DNA damage |
Tail length(μm) |
% of DNA in tail |
Tail moment (μm) |
|
|
Un- Sporulated Oocysts |
5 ppm/24h |
70.8±0.8bc |
12.2± 0.3d |
7.6± 0.5d |
6.5±0.6d |
0.6 ± 0.02c |
| 10 ppm/24h |
78.2±0.9b |
14.3± 0.8bc |
8.3 ± 0.6c |
8.34 ± 0.5c |
0.62 ± 0.04c |
|
| 20 ppm/24h |
86.3±0.84ab |
16.44± 0.55b |
9.5 ± 0.5c |
9.7±0.7c |
0.86 ±0.02ab |
|
| 40 ppm/24h |
94.8±1.3a |
18.3 ± 0.81a |
10.3 ± 0.7ab |
10.7 ±0.5ab |
0.91 ±0.01a |
|
| Sporulated oocysts | 20ppm/28h | LC 90 % |
17.8 ± 0.62a |
9.7 ± 0.9ab |
9.9 ±0.7b |
0.88 ±0.03ab |
| 40ppm/15h | LC90% |
18.2 ± 0.4a |
11 ± 0.26a |
11.9 ±0.5a |
0.92 ±0.04a |
|
| Amprolium 240 ppm/24h |
98.7 ±0.9a |
18.8± 0.5a |
11.6 ± 0.56a |
12±0.25a |
0.98 ±0.08a |
|
| Negative control | 0.0 | 4.2 ±0.4 | 3.7 ±0.4 | 3.43± 0.4 | 0.46± 0.03 | |
| P-value |
≤ 0.05 |
|||||
* Data represented as mean ± SD.
*a-c Means with different superscripts within the same column are significantly (p-value ≤ 0.05) different.
lium 240 ppm/24 h) and of negative control non-exposed oocysts (Table 5 and Figure 5).
The data demonstrated a direct relationship between the increase in the dose of n curcumin and the degree of the overall DNA genotoxic damage as it is also represented by variations in the tail length (μm), % of DNA in the tail segment, and tail moment (μm). When investigating the migration of DNA fragments by agarose gel electrophoresis, an increase in the % of sporulation inhibition from 70.8 ± 0.8 to 94.8 ± 1.3 was observed with increasing the curcumin dose from 5 ppm to 40 ppm, respectively. A significant increase (p ≤ 0.05) in the % of DNA damage was also detected from 12.2± 0.3 to 18.9 ± 0.4, the mean tail length (μm) increased from 7.6 ± 0.5 to 11 ± 0.26, the % of DNA in the tail was increased from 6.5 ± 0.6 to 11.4 ± 0.5, and the tail moment (μm) was also increased with increasing the concentration from 0.6 ± 0.02 at 5 ppm to 0.95 ± 0.04 in oocysts exposed to 40 ppm. The values gradually increased with the tested concentrations from 5 to 40 ppm (Table 5). The same facts were also recorded in sporulated oocysts exposed to LC90.
The mean values of destruction in DNA parameters in unsporulated oocysts exposed to 40 ppm and in sporulated ones exposed to LC90 have no significant difference (p ≤ 0.05) compared to that recorded after exposure to the control reference drugs (amprolium 240 ppm). At the same time, significant very low destruction (4.2 ± 0.4%) in DNA and the other estimated parameters were recorded in control non-exposed oocysts (Table 5 and Figure 5).
DISCUSSION
Medicinal plants and potential herbs are still very widely exposed for improvement to be used in the field of human, animal, and poultry health, especially those that have bioactive functions such as antimicrobial, antioxidant, anti-diabetics, antiparasitic, anti-cancerous, and other functions (Haniarti et al., 2019; Jamil et al., 2022). There is a general increasing demand to reduce applying antibiotics in poultry farms and replace them with new natural alternatives (Raza et al., 2022). Curcumin extracted from Curcuma longa herbal plant is widely investigated in this respect as it has anti-inflammatory, antimicrobial, antiproliferative, neuroprotective and wound healing properties for humans and animals (Willenbacher et al., 2019).
It was recorded that curcumin has anti-protozoal and antioxidant activity, effectively reducing coccidia and other poultry protozoal infections (Abbas et al., 2010). Curcumin possesses many medical benefits but must be used at high concentrations (10 g/L) to induce considerable sporulation inhibition to the immature Eimeria spp. oocysts (El-Khtam et al., 2014; Yadav et al., 2020). Moreover, crude curcumin is unstable in the working solution and degrades during long-timed sample preparation (Ajay et al., 2012).
For these causes, the direction towards developing and manufacturing nano-curcumin was performed to gain the benefits of curcumin after using a low dose level and small-sized particles with higher stability. In this respect, the present study was initiated to evaluate the anti-coccidial effects of native original newly formulated nano-curcumin molecules after loading at particles of olive oil.
C-Oo.Nc was produced in Departement of Pharmacology Faculty of Veterinary Medicin Cairo University, Giza, Egypt. Characterization of the produced C-Oo.Nc using TEM, SEM, and AFM revealed that the obtained particles are spherical structures with maximum thickness less than 50 nm. Moreover, it was noticed that C-Oo.Nc particles do not tend to form agglomeration in certain areas. Zeta size and potential values demonstrated that the produced molecules are 63 nm sized with good stability in the aqueous media. The obtained particle size was within the range previously described for the suitable size of nanoparticles. These characteristics are identical to the findings of (Kamel et al., 2019) who characterized and described the successful nanoparticles characters.
In this study, anti-coccidial effect of the produced C-Oo.Nc was evaluated in Department of Parasitology, Faculty of Veterinary Medicine, Cairo University, Egypt. The current study evaluated the inhibitory effects of C-Oo.Nc on un-sporulated and sporulated oocysts of five Eimeria spp.; aiming to determine C-Oo.Nc LC50 and LC90 to be used in the second part of the study versus infected chicken in vivo. Efficacy of the product was evaluated by measuring its sporulation inhibitory % of unsporulated oocysts, while the sporulated oocysts exposed to the calculated LC50 & LC90 were evaluated after inoculation to groups of broiler chickens. In addition, DNA damage of the exposed and control oocysts was also evaluated using the comet assay.
The current study proved that C-Oo.Nc inhibited the sporulation ability of the exposed immature Eimeria spp. oocysts. This increased with the increase in C-Oo.Nc concentration and exposure time. This was in agreement with (El-Banna et al., 2005; Ramadan et al., 1997; El-Khtam et al., 2014). The mean rate of inhibition was increased from 9.82 ±0.88 % after exposure of the oocysts to 5 ppm/1h to reach 77.50±0.75 % after 36 h exposure time. Also, the % of sporulation inhibition in oocysts exposed to 10ppm was increased from 28.66 ±0.97% to 85.24±1.25 % with an increasing the exposure time from 1h to 36h Exposure to 20 ppm/1h revealed 36.0 ± 1.5 % sporulation inhibition rate increased to 97,33±1.7 % after 36 h. The maximum inhibitory effects were recorded after exposure to 40 ppm/1h as it reached 42.0± 1.2% increased to 95 then to 97.66±0.95 % after 48h & 36 h respectively. Increasing the inhibitory rate of sporulation in exposed Eimeria spp. oocysts In vitro agreed with that previously described by Felici et al., (2021).
From the previous screening, the calculated LC50 was 5.0 ppm/9 h, 10 ppm/5 h, 20 ppm/4 h, or 40 ppm/90 minutes of exposure time. The LC90 was recorded after exposure to 20 ppm and 40 ppm only after 28 and 15 hours of exposure time, respectively. These results agreed with Felici et al. (2021). The recorded high efficacy of the tested C-Oo.Nc at the lower concentrations has come in agreement with Gogoi et al. (2019) who reported that nano-curcumin has a great anti-coccidial effect on the experimentally infected broiler chicks at low doses. Moreover, Tsai et al. (2011) reported that the nano form of curcumin is easily dissolvable in water and has a significantly higher penetration and distribution rate. In addition, Partovi et al. (2019) conducted that dietary supplementation with nanocurcumin at low doses is safe and effective on carcass characteristics and physical and chemical properties of broiler chicken breast meat infected with Eimeria acervulina, Eimeria maxima, and Eimeria tenella.
However, these results were contrary to that mentioned by El-Khtam et al. (2014), as they obtained 60% inhibition in sporulation of the exposed oocysts at a very high dose (0.8 to 10 g/L during 6-18 hours of exposure with no significant difference between the different concentrations and exposure time. This may be due to variations in the level of the solubility of the type of curcumin they evaluated. Moreover, Curcumin’s biological action is dependent on its bioavailability. Curcumin bioavailability was affected by the amount and concentration at which it is absorbed, occurs in the plasma, and reaches its target area (Paulraj et al., 2019).
The recorded efficacy of newly formed C-Oo.Nc on the level of this study gives this formulation special superiority related to induction of good target effect at low concentrations. On the contrary of this, nano-chemicals such as nano-silver materials proved potent effects at low doses than the other similar nano-plant extracts such as nano-curcumin, but the nano chemicals usually have a narrow safety margin than that of plant one (Elnabarawy et al., 2020; Madbouly et al., 2022).
The damage in DNA of the exposed and control Eimeria spp oocysts was evaluated using the comet assay on immature oocysts exposed to the tested four concentrations of C-Oo.NC after 24 h exposure time, for sporulated oocysts exposed to LC90 (20 ppm/26 h & 40 ppm/15 h) as well as in control non-exposed oocysts. Comet assay revealed a direct relationship between the increase in the dose of C-Oo.NC and the degree of the overall DNA genotoxic damage which is represented by variations in the tail length (μm), % of DNA in the tail segment, and tail moment (μm). Investigating the migration of DNA fragments by agarose gel electrophoresis revealed no significant difference (p ≤ .05) in the mean values of destruction in DNA parameters in unsporulated oocysts exposed to 40 ppm and sporulated ones exposed to LC90 as well as that exposed to the control reference drugs. Moreover, significant very low destruction (2.30±0.31) in DNA and the other estimated parameters were recorded in the control non-exposed oocysts. The ability of nano-curcumin to induce DNA damage in the exposed agents was previously described by Kumar et al. (2015) and Attaullah et al. (2020) as they mentioned that changes in the tail length investigated by comet assay in comparison with the control non-exposed stages was reflected marked DNA damage and this damage was increased by the increase in the concentration of tested curcumin extract.
To investigate the effect of the C-Oo.Nc on the exposed sporulated oocysts. The sporulated oocysts exposed to the previously estimated LC50 & LC90 were inoculated to separate groups of 14th days old broiler chickens. The results revealed a marked decrease in the mean total number of oocysts shed from each bird during the whole period (5th to 10th dpi) (2200 ± 50 and 1580 ± 50 ) in the group inoculated by oocysts exposed to both calculated LC90 (20 ppm/28 h and 40 ppm/15 h) as well as a significant decrease (p ≤ 0.05) in the mean total number of shed oocysts in groups exposed to LC50 (11520 ± 120 to 10080 ± 100) in comparison with that shed from control birds (33680 ± 150) inoculated by un-exposed sporulated oocysts. This decrease in pathogenicity of the exposed sporulated oocysts was clearer at PM inspection performed at the end of the experiment (12th dpi) as very slight or un-apparent reddens without thickening in the wall of ceci. This was closely related to the normal un-infected control was recorded in the group inoculated by oocysts exposed to LC90. This was in contrast to the group inoculated by sporulated oocysts exposed to LC50. The direct relation between infectivity inhibition and the degree of the produced lesion score after exposure to different concentrations of the tested drugs was in agreement with that recorded in previous works of Ramadan et al. (1997) and El-Banna et al. (2005) on other anti-coccidial drugs. The ability of the used medical plants to inhibit the infectivity of the exposed oocysts was previously mentioned by several authors, for example, Tsai et al. (2011) and El-Khtam et al. (2014), as a clear sign of the success of treatment and control of the disease. It is worth mentioning that the marked severe effect of PM was observed in control birds in ceca due to increasing the % of E.tenella oocysts in the original inoculum (Ramadan et al. 1997; El-Banna et al. (2005).
CONCLUSION
An original new formulation of nano-curcumin on olive oil base (C-Oo.Nc) was prepared, evaluated, and investigated. The present study proved that this new formulation has an effective, safe, economic coccidiocidal effect in low concentrations which able to induce severe genotoxic damage on the DNA level of the zygote and its sporocysts. It markedly inhibited the sporulation and infection of mature and immature Eimeria spp. oocysts. The authors of the present study are currently performing an experimental In vivo study on larger groups of chickens to apply more investigations to evaluate the efficacy of C-Oo.Nc as a coccidiocidal product regarding to different aspects such as chickens’ health, body weight, immunity, biochemical parameters, histopathological study and other factors.
ETHICS APPROVAL
All steps of the applied protocol followed the guidelines demonstrated by the Faculty of Veterinary Medicine and the Institutional Animal Care and Use Ethical Committee of Cairo University Ethics Committee, Giza, Egypt (VetCU10102019097).
ACKNOWLEDGMENT
The authors thank technical staff or assistance during collection of samples.
CONFLICT OF INTEREST
All authors declare that they have no conflict of interest and the work was self-funded.
AUTHORS’ CONRIBUTIONS
All the authors shared the aim of the study. Mohamed M. El-Bahy, Shimaa Abdel-Radi, Reem M. Ramadan performed the steps of the current protocol and interpreted the results. Where, Fady Sayed Youssef, Gehad Genidy Mohamed, Sameh Hamed Ismail produced and characterized curcumin nanoparticles. All authors drafted, critically revised, and approved this manuscript.
REFERENCES
Abbas RZ, Iqbal Z, Khan MN, Zafar MA, Zia MA (2010). Anticoccidial activity of Curcuma longa L. in Broiler Chickens. Brazilian Archiv. Biol. Technol., 53 (1): 63-67. http://dx.doi.org/10.1590/S1516-89132010000100008
Abdel-Rahman GH, Abdel-Radi S (2022). Synergistic action of Viteselen with anti-Fasciola drug as a tool for improving fertility and hemato-biochemical biomarkers in Fasciola infected sheep. J. Parasit. Dis. DOI: https://www.doi.org/10.1007/s12639-021-01423
Abou-Okada M, Abu-Bakr HO, Hassan A, Abdel-Radi S, Aljuaydi SH, Abdelsalam M, Taha E, Younis NA, Abdel-Moneam DA (2021). Efficacy of Acriflavine for controlling parasitic diseases in farmed Nile tilapia with emphasis on fish health, gene expression analysis, oxidative stress, and histopathological alterations. Aquaculture., 541 (736791): 1-11. https://doi.org/10.1016/j.aquaculture
Ajay S, Harita D, Tarique M, Amin P (2012). Solubility and dissolution rate enhancement of curcumin using kollidon VA64 by solid dispersion technique. Int. J. Pharm. Tech. Res., 4:1055-1064. https://sphinxsai.com/2012/july_sept12/pharm/pdfpharm/PT=23(1055-1064)%20JS%2012.pdf
Attaullah MKZ, Zahoor MA, Mubarik MS, Rizvi H, Majeed HN, Zulhussnain M, Ranian K, Sultana K, Imran M, Qamer S (2020). Insecticidal, biological and biochemical response of Musca domestica (Diptera: Muscidae) to some indigenous weed plant extracts. Saudi J. Biolog. Sci. 27:106–116. https://doi.org/10.1016/j.sjbs.2019.05.009
Chand N, Faheem H, Khan RU, Qureshi MS, Alhidary IA, Abudabos AM (2016). Anticoccidial effect of mananoligosacharide against experimentally induced coccidiosis in broiler. Environ. Sci. Pollut. Res., 23(14):14414-14421. https://doi.org/10.1007/s11356-016-6600-x
El-Banna HA, El-Bahy MM, El-Zorba HY, Hady M (2005). Anticocidial Efficacy of Drinking Water Soluble Diclazuril on Experimental and Field Coccidiosis in Broiler Chickens. J. Vet. Med. A. (Blackwell Verlag, Berlin) 52: 287-291. https://doi.org/10.1111/j.1439-0442.2005.00727.x
Elgadir MA, Uddin MS, Ferdosh S, Adam A, Chowdhury AJK, Sarker MZI (2015). Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: A review. J. Food Drug Anal. 23: 619-629. https://doi.org/10.1016/j.jfda.2014.10.008
El-Khtam AO, Abd El Latif A, El-Hewaity MH (2014). Efficacy of turmeric (Curcuma longa) and garlic (Allium sativum) on Eimeria spp in broilers. Int. J. Basic Appl. Sci., 3 (3): 349-356. https://doi.org/10.14419/ijbas.v3i3.3142.
Elnabarawy AM, Khalifa MM, Shaban KS, Kotb WS (2020). Evaluation of the Effect of Mycotoxins in Naturally Contaminated Feed on the Efficacy of Preventive Vaccine against Coccidiosis in Broiler Chickens. J. World Poult. Res. 10(2S): 235-246. DOI: https://dx.doi.org/10.36380/jwpr.2020.29
Esatbeyoglu T, Huebbe P, Ernst IM, Chin D, Wagner AE, Rimbach G (2012). Curcumin-from molecule to biological function. Angewandte Chemie International Edition, 51(22): 5308-5332. https://doi.org/10.1002/anie.201107724
Fatemi A, Razavi SM, Asasi K, Torabi Goudarzi M. (2015). Effects of Artemisia annua extracts on sporulation of Eimeria oocysts. Parasitol. Res., 114(3):1207-1211. https://doi.org/10.1007/s00436-014-4304-z
Felici M, Tugnoli B, Piva A, Grilli E (2021). In vitro Assessment of Anticoccidials: Methods and Molecules Animals (Basel).) 11(7): 1-19. https://doi.org/10.3390/ani11071962
Gogoi C, Sarma J, Barua CC, Tamuly S, Upadhyaya TN, Islam S, Sonowal J, Borthakur U, Banerjee DK, Barkathullah N (2019). Evaluation of nano-curcumin on experimentally induced coccidiosis in broiler chicks. IJCS, 7(3):4514-4520. https://www.chemijournal.com/archives/2019/vol7issue3/PartBV/7-3-496-319.pdf
Hafez HM (2008). Poultry coccidiosis: Prevention and control approaches. Archiv. Fur Geflugelkunde, 72(1):2-7. https://www.european-poultry-science.com/Poultry-coccidiosis-prevention-and-control-approaches, QUlEPTQyMTg3ODEmTUlEPTE2MTAxNA.html
Haniarti M, Akib MA, Ambar A, Rusman ADP, Abdullah A (2019). Herbal for increasing immunity and weight of poultry. Earth Environ. Sci., 247:(012056):1-7. https://doi.org/10.1088/1755-1315/247/1/012056
Jamil M, Aleem MT, Shaukat A, Khan A, Mohsin M, Rehman TU, Abbas RZ, Saleemi MK, Khatoon A, Babar W (2022). Medicinal Plants as an Alternative to Control Poultry Parasitic Diseases. Life, 12(449): 1-13. https://doi.org/ 10.3390/life12030449
Kamel AE, Fadel M, Louis D (2019). Curcumin-loaded nanostructured lipid carriers prepared using Peceol™ and olive oil in photodynamic therapy: development and application in breast cancer cell line Int. J. Nanomed., 14: 5073–5085. https://doi.org/10.2147/IJN.S210484
Khalafalla REU, Müller M, Shahiduzzaman V, Dyachenko AY, Desouky G, Daugschies A (2011). Effects of curcumin (diferuloylmethane) on Eimeria tenella sporozoites in vitro. Parasitol. Res., 108 (4): 879-886. http://dx.doi.org/10.1007/s00436-010-2129-y.
Kocaadam B, Sanlier N (2017). Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. 57:2889-2895. https://doi.org/10.1080/10408398.2015.1077195
Koide T, Nose M, Ogihara Y (2002). Leishmanicidal Effect of Curcumin in Vitro. Biol. Pharm. Bull. 25(1):131–133. https://doi.org/10.1248/bpb.25.131
Kumar A, Sharma S, Verma G (2015) Insecticidal and genotoxic potential of Acorus calamus rhizome extract against Drosophila melanogaster. Asian J. Pharm. Clin. Res. 8:113–116. https://doi.org/10.13102/sociobiology.v64i4.1867
Lillehoj HY, Liu S, Calsamiglia ME, Fernandez-Miyakawa F, Chi RL, Cravens SO, Gay CG (2018). Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet. Res. 49 (76):1-18. https://doi.org/10.1186/s13567-018-0562-6
Madbouly NT, Abdel-Radi S, Youssef FM, Auda HM, El-Bahy MM, Ramadan RM (2022). Parasiticidal Efficacy of a New Formulation of Silver nanoparticles Parasiticidal Efficacy of a New Formulation of Silver nanoparticles on Trichinella spiralis in vitro. J. Adv. Vet. Res., 12(4). In Press. https://advetresearch.com/index.php/AVR/issue/view/47
Mesa-Pineda C, Navarro-Ruíz JL, López-Osorio S, Chaparro-Gutiérrez JJ, Gómez-Osorio LM (2021). Chicken Coccidiosis: From the Parasite Lifecycle to Control of the Disease. Front. Vet. Sci., 8: 1-15. https://doi.org/10.3389/fvets.2021.787653
Nagajyothi FD, Zhao LM, Weiss HB, Tanowitz (2012). Curcumin treatment provides protection against Trypanosoma cruzi infection. Parasitol. Res. 110(2491): 1-15. https://doi.org/10.1007/s00436-011-2790-9.
Partovi R, Seifi S, Pabast M, Babaei A (2019). Effects of dietary supplementation with nanocurcumin on quality and safety of meat from broiler chicken infected with Eimeria species. J. Food Safety. 39(6): 1-9. https://doi.org/10.1111/jfs.12703
Paulraj F, Abas FH, Lajis N, Othman I, Naidu R (2019). Molecular Pathways Modulated by Curcumin Analogue, Diarylpentanoids in Cancer. Biomolecules. 9: 270. https://doi.org/10.3390/biom9070270
Petrie A, Watson P (1999). Statistics for Veterinary and Animal Science. 1st Ed. The Black well Science Ltd, United Kingdom, 90-115. https://www.agrifs.ir/sites/default/files/Statistics%20for%20Veterinary%20and%20Animal%20Science%2C%20Third%20Edition-www.gkambiz.blogfa.com_.pdf
Pop LM, Varga E, Coroian M, Nedișan ME, Mircean V, Dumitrache MO, Farczádi L, Fülöp I, Croitoru MD, Fazakas M, Gyӧrke A (2019). Efficacy of a commercial herbal formula in chicken experimental coccidiosis. Parasit. Vectors., 12(1):1-9. https://doi.org/10.1186/s13071-019-3595-4
Ramadan A, Abo El-Sooud K, El-Bahy MM (1997). Anticoccidial efficacy of toltrazuril and halofuginone against Eimeria tenella infection in broiler chickens in Egypt. Res. Vet. Sci., 62:175-178. https://doi.org/10.1016/S0034-5288(97)90142-9.
Raza QS, Saleemi MK, Gul S, Irshad H, Fayyaz A, Zaheer I, Tahir MW, Fatima Z, Chohan TZ, Imran M (2022). Role of essential oils/volatile oils in poultry production—A review on present, past and future contemplations. Agrobiol. Rec. 2022, 7:40–56. https://agrobiologicalrecords.com/articles/3-1-Vol-7-40-56-2022-ABR-21-0875.pdf
Salama MM, Taher EE, El-Bahy MM (2012). Molluscicidal and Mosquitocidal Activities of the Essential oils of Thymus capitatus L. and Marrubium vulgare L. American J. Drug Discov. Develop.; 2 (4): 204-211. https://doi.org/10.1590/S0036-46652012000500008
Santos HM, Tsai CY, Catulin GEM, Trangia KCG, Tayo LL, Liu HJ, Chuang KP (2020). Common bacterial, viral, and parasitic diseases in pigeons (Columba livia): A review of diagnostic and treatment strategies. Vet. Microbiol., 247(108779):1-13. https://doi.org/10.1016/j.vetmic.2020.108779
Singh NP, McCoy MT, Tice RR, Schneider EL (1988). A simple technique for quantitation of low levels of DNA damage in Individual cells. Exp. Cell Res. 175(1):184–191. https:// doi. org/ 10. 1016/ 0014- 4827(88) 90265-0
Teng PY, Castro F, Kim WK (2021). Nutrition and Coccidiosis. In Proceedings of the Arkansas Nutrition Conference, 1(3): 1-11. https://scholarworks.uark.edu/panc/vol2021/iss1/3
Tsai YM, Chien CF, Lin LC, Tsai TH (2011). Curcumin and its nano-formulation: the kinetics of tissue distribution and blood–brain barrier penetration. Int. J. Pharmaceut., 416(1): 331-338. https://doi.org/10.1016/j.ijpharm.2011.06.030
Willenbacher E, Khan SZ, Mujica SCA, Trapani D, Hussain S, Wolf D (2019). Curcumin: New Insights into an Ancient Ingredient against Cancer. Int. J. Mol. Sci. 20(8)1808: 1-13. https://doi.org/10.3390/ijms20081808
Wunderlich F, Al-Quraishy S, Steinbrenner H, Sies H, Dkhil MA (2014). Towards identifying novel anti-Eimeria agents: trace elements, vitamins, and plant-based natural products. Parasitol. Res., 113(10): 3547-3556. https://doi.org/10.1007/s00436-014-4101-8
Yadav S, Jha R (2019). Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol., 10(1):1-11. https://doi.org/10.1186/s40104-018-0310-9
Yadav S, Teng PY, Santos TS, Gould RL, Craig SW, Fuller AL, Pazdro R, Kim WK (2020).The effects of different doses of curcumin compound on growth performance, antioxidant status, and gut health of broiler chickens challenged with Eimeria spp. Poult. Sci. 99:5936–5945. https://doi.org/10.1016/j.psj.2020.08.046.
Youssef F, Mohamed G, Ismail S, Elzorba H, Galal A, Elbanna H (2021). Synthesis, Characterization and In vitro Antimicrobial Activity of Florfenicol-Chitosan Nano-composite, Egypt. J. Chem., 64: 941 – 948. https://doi.org/10.21608/EJCHEM.2020.43238.2883
To share on other social networks, click on any share button. What are these?





