Assessment of Earias vittella (Fabricius) Egg Distribution in Cotton Crop at Multan, Pakistan: A Comprehensive Guideline for Developing a Pest Scouting Method
Assessment of Earias vittella (Fabricius) Egg Distribution in Cotton Crop at Multan, Pakistan: A Comprehensive Guideline for Developing a Pest Scouting Method
Muhammad Tahir Jan1, Sarfraz Ali Shad2, Mushtaq Ahmad Saleem3 and Muhammad Binyameen2,*
1Entomology Section, Central Cotton Research Institute, Old Shujabad Road, 60800 Multan
2Departments of Entomology, Faculty of Agricultural Sciences and Technology, Bahauddin Zakariya University, 60800 Multan
3Faculty of Life Sciences, University of Central Punjab, Lahore
ABSTRACT
The knowledge of oviposition preference by female insects is a basic requirement for developing insect pest scouting methods to determine the potential damage and to apply control measures for key pest like Earias vittella in cotton. Field experiments were conducted to assess the distribution of E. vittella eggs within the cotton plant. Out of 240 plants inspected, E. vittella females laid 204 and 194 eggs on 40.9% and 36.9%of the plants in 2011 and 2012, respectively. Spearman Rank Correlation showed that a significant and positive relationship (association) existed between the number of infested plants or plant parts and number of eggs laid. In every week, an average of 6.1±1.2 plants were infested with13.6±2.7 eggs in 2011 and 5.5±1.1 plants with 12.9±2.2 eggs in 2012. Four and three peak egg-laying periods were observed in 2011 and 2012, respectively. Taylor’s power law of regression showed an aggregate spatial pattern distribution of eggs for E. vittella in plants in both years. Variance-to-mean ratio, the chi-square test index, and Lloyd index of patchiness indicated uniform eggs distribution for both years. Within the plants, the most preferred oviposition sites were just below the terminal portion on such structures as the leaves, terminal buds and NE leaves. The current study regarding the site selection for oviposition provides information that can be used to develop guidelines for E. vittella management in cotton production.
Article Information
Received 22 February 2019
Revised 23 May 2020
Accepted 13 February 2021
Available online 15 June 2021
(early access)
Published 25 February 2022
Authors’ Contribution
MTJ, SAS, MAS and MB planned the study. MTJ conducted the filed experiments. MTJ, SAS and MB did the statistical analysis. MTJ wrote the article with input from all co-authors.
Key words
Egg-laying, Main stem leaves, Oviposition, Site preference, Sympodial branches.
DOI: https://dx.doi.org/10.17582/journal.pjz/20190222180222
* Corresponding author: mbinyameen@bzu.edu.pk
0030-9923/2022/0003-1303 $ 9.00/0
Copyright 2022 Zoological Society of Pakistan
Introduction
The spotted bollworm, Earias vittella Fab (Lepidoptera: Noctuidae), is an important pest of cotton worldwide. It is widely distributed in Pakistan and India where it causes huge damage to cotton (Aziz et al., 2012). E. vittella infests different parts of cotton plants leading to shedding of squares, flowers and bolls (Dhawan et al., 1990), resulting in about 50% losses in cotton seed yield (Sidhu and Sandhu, 1977; Hasan and Ansari, 2010). The pest remains active throughout the year on different alternate hosts and produces 6-8 generations (Vennila et al., 2005). In Pakistan, the initial attack by E. vittella has been observed on cotton during June and July, with maximum infestation occurring during August and September (Syed et al., 2011).
Various attempts have been made in the past to control E. vittella under integrated pest management (IPM) strategies such as by cultivation of resistant cultivars (Sharma and Agarwal, 1983; Drake, 1991; Dhillon and Sharma, 2004), application of non-host plant extracts, use of bio-control agents and applications of bio-rational and conventional insecticides (Aziz, 2010; Badiyala, 2013; Rahman et al., 2015). An important reason for the failure of E. vittella control strategies is poor understanding of the ecological linkage between the pest and its host. Site preference for egg laying/oviposition is an important ecological interaction between phytophagous insects and their host plants and therefore depends on host quality (Batáry et al., 2008). Such factors can lead to variation in performance and survival of insect progeny for eggs deposited in different locations within a plant (Holland et al., 2004). A study of the vertical distribution of eggs within plant aimed at the determining their location in the plant is crucial to minimize the time spent during the sampling process and augments the reliability of the IPM (Renwick and Chew, 1994). Despite the economic importance of E. vittella moth, its host selection and within-plant distribution are poorly understood.
The knowledge of oviposition site selection is a basic requirement in developing a pest monitoring strategy (Ross and Ostlie, 1990) and the application of control measures. Several reports on within-plant distribution of different lepidopteran insects like Heliothis spp. (Wilson et al., 1980; Duffield and Chapple, 2001), Alabama argillacea (Hübner) (Fernandes et al., 2007) in cotton, Grapholitha packardii (Zeller) in blue barriers (Mallampalli and Isaacs, 2002) and leaf miner, Tutaabsoluta (Meyrick) in processing tomatoes (Torres et al., 2001) are available.
Thus, knowledge about E. vittella site selection with in the cotton plant is needed in order to develop an acceptable sampling programme and control plan for this pest. Chakravarthy (1985) and Sharma and Agarwal (1983) recommended the whole plant as a sampling unit for eggs assessment. Though theoretically feasible, one would spend several hours in sampling for eggs on whole plants in the field. Therefore, within-plant eggs distribution is an important characteristic of ecological communities that provide farmers with guidelines to control E. vittella (Debouzie and Thioulouse, 1986; Arbab and McNeill, 2014). The egg distribution for other cotton bollworms was adequately described by the spatial distribution (Nachapong, 1980). Analysis of the spatial distribution of insects allows for an estimation of pest densities and identifies key timing for pest management decisions that allow growers to minimize costs of control (Khaing et al., 2002).
Therefore, current information for the assessment of eggs within the plant as well as within the field is of utmost importance to monitor E. vittella population in the crop. The present study described how the eggs were distributed within cotton plants (intra plant distribution), a critical aspect in devising effective control measures against the pest.
Materials and methods
Field experiments were conducted at the Central Cotton Research Institute [30°12’N latitudes and 71°26’E longitudes and 123 m altitude/elevation], Multan, Pakistan, with plot position as North-South over two seasons (2011 and 2012) to collect data about the preferred oviposition sites of E. vittella in unsprayed cotton crops. The commercial cotton variety, CIM-496, was sown during the 2nd week of May with row to row and plant to plant distances of 76 cm and 22.9 cm, respectively. The cotton was planted to three plots (representing three replications) each measuring 30 m × 10 m. Experimental plots were separated by 2 m wide fallows. Standard agronomic practices were performed in growing the cotton crop.
Data on egg-laying (No. of eggs), was recorded on a weekly basis starting from the 1st week of July (about 45 days after sowing) till the last week of October during both years on: (i) main stem leaves (MSL) (at base of sympodial branch), sympodial branches (S. Br) and (ii) Terminal bud (Ter. bud), newly emerged leaves (NE), leaves (all types), squares, flowers and bolls.
During every sampling, 15 plants (N=15) were randomly selected and examined individually for eggs. Egg-counting was done with the aid of a 10 X magnifying glass.
Percent increase in S. Br over MSL was calculated as follows:
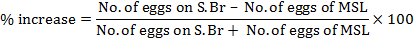
Number of eggs on each leaf node of MSL and on each S. Br was total egg found on respective part during whole season.
Statistical analysis
Data on egg counts was analyzed by General linear Model by using Statistix 8.1 (Statistix, 2008). The Least significant difference (LSD) was used separate the means of various parameters used for eggs and larval population on the cotton plant (Gomez and Gomez, 1984).
One sample T-test with Spearman Rank Correlation for number of eggs and infested plants within the plant, Taylor’s power law based on regression techniques (Taylor, 1984), index of dispersion (ID) (Elliott, 2003) and index of patchiness (IP) (Lloyd, 1967) were used to test the dispersion of the pest because the use of a single index can lead to incorrect conclusions (Myers, 1978).
Spearman rank correlation
The Spearman’s rank-order correlation is the nonparametric version of the Pearson product-moment correlation. Spearman’s correlation coefficient, (ρ, also signified by rs) measures the strength and direction of association between two ranked variables likewise total number of eggs and the infested cotton plants within field (Fauvergue et al., 1994).
Taylor’s power law based on regression techniques
The Taylor’s power law based on variance-mean relationships, (s2=axb) has been used widely (Arbab and McNeill, 2014) to infer the type of spatial distribution (uniform, random or aggregated) of the counts of E. vittella eggs and larval population in the cotton field. The equation is solved with linear regression as:
Log (s2) = log (a) - blog(x)
Where, s2 and x are the sample variance and sample mean, respectively; the slope (b) is a measure of aggregation (dependent upon species behavior or the environment); and the intercept (a) is a scaling factor. When a=b=1, a>1, b<1, and a<1, b>1, the distribution is random, uniform, and aggregated, respectively (Ifoulis and Savopoulou-Soultani 2006).
Index of dispersion, ID = S2 / ẍ; (where S2 is sample variance and ẍ is sample mean) is known as the variance to-mean ratio is commonly considered to be a useful index to describe the spatial distribution of a given insect within the field. Where ID=1 population is random, <1 regular, and >1 aggregated (Kuno, 1991).
Modified the equation performing chi-square test for the variance-to-mean ratio to determine its significant departure from 1.0 as:
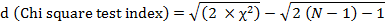
Whereas, Chi-square, χ2= (S2/ẍ) × (N-1).
If d<1.96, agreement with a random distribution and <−1.96, a uniform distribution, and if d>1.96, an aggregated distribution is suspected.
The index of patchiness:
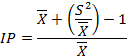
This index was proposed by Lloyd (1967) to measure the pattern intensity of the pest that is unaffected by thinning (the random removal of points) or plants density variation. If IP=1, random, <1 uniform, and >1 aggregated.
Results
Oviposition site preference for sympodial branches and main stem leaves
A total of 240 plants were observed during 2011 and 2012. E. vittella females oviposited 204 eggs on ninety-two plants (40.9%) in 2011 and 194 eggs on eighty-three plants (36.9%) in 2012. Test for association between the number of infested plants and eggs laid as determined with help of SRC (Spearman rank correlation) showed significant relationship 2011: ρ=0.876; P-value=0.00and 2012: ρ =0.969; P-value =0.00forthe females to select the plants for egg laying. The female moth selected 6.1 ± 1.2 plants for 13.6 ± 2.7 eggs per week in 2011 and 5.5 ± 1.1 plants for 12.9 ± 2.2 eggs in 2012 (Table I). During the crop growing season, four peak egg laying periods (two each in August and September in 2011 and three, one in August and two in September in 2012) were observed (Fig. 1).
Table I reveals that the number of eggs laid on MSL (main stem leaves) and S. Br (Sympodial branches) varied significantly (F (1, 27) = 10.3 and P-values= 0.0034, respectively in 2011 and F (1, 27) =20.8; P- value =0.0001 in 2012). The females laid 3.6 ± 0.72 (26.47%) and 2.9 ± 0.52 (22.16%) eggs on MSL of 2.3 ± 0.49 (15.6%) and 2.3 ± 0.42 (15.1%) plants in 2011 and 2012 per week, respectively. Association between MSL and number of plants ρ =0.65; P-value= 0.01 (2011) and 0.86; P-value= 0.00 (2012) was significantly positive. The results further revealed that an average of 10.0 ± 2.49 eggs per 6.1 ± 1.20 plants in 2011 and 10.1 ± 1.84 eggs per 5.5 ± 1.08 plants, were distributed among S. Br of infested plants. Positively high SRC, (2011: ρ =0.88; P-value=0.00 and 2012: 0.95; P-value=0.00) for sympodial branches also reveal female moth selection of fruiting branches for oviposition in the field (Table I).
Seasonal variation in E. vittella eggs laying
Significant seasonal variation in egg-laying on MSL (2011; (F (3, 12) =8.02; P- value =0.0034 and 2012; F (3, 12) =4.39; P-value=0.027) and S. Br (2011; (F (3, 12) =13.26; P- value =0.0004 and 2012; F (3, 12) =7.4; P- value = 0.0046) was observed. The most eggs laid on S. Br (20.8 and 13.5 in 2011 and in 13.5&15.5 in 2012 per week) and MSL (3.5 and 7.0 in 2011 and 4.0 and 4.3 in 2012) were recorded in August and September (Table II).
Table I.- Number of infested plants out of 240 plants inspected, number of eggs of E. vittella during each season and Spearman Rank Correlation of infested plants with eggs.
|
Year |
No. of infested plant |
No. of eggs |
*SRC (ρ) |
|||
|
Total (%) |
Mean No. of infested plants/week |
Total (%) |
Mean No. of eggs/week |
|||
|
2011 |
Total |
92 (40.9) |
6.1 ± 1.20 |
204 |
13.6 ± 2.70 |
0.88; P-value = 0.00 |
|
MSL |
(15.6) |
2.3 ± 0.49 |
(26.47) |
03.6 ± 0.72 |
0.65; P-value = 0.01 |
|
|
S. Br |
(40.9) |
6.1 ± 1.20 |
(73.53) |
10.0 ± 2.49 |
0.88; P-value = 0.00 |
|
|
2012 |
Total |
83 (36.9) |
5.5 ± 1.10 |
194 |
12.9 ± 2.20 |
0.97; P-value = 0.00 |
|
MSL |
(15.1) |
2.3 ± 0.42 |
(22.16) |
02.9 ± 0.52 |
0.86; P-value = 0.00 |
|
|
S. Br |
(36.9) |
5.5 ± 1.08 |
(77.84) |
10.1 ± 1.84 |
0.95; P-value = 0.00 |
|
*SRC, Spearman rank correlation.
Table II.- Mean number of eggs/week laid by E. vittella females on MSL and S. Br of 15 plants during different months in 2011 and 2012.
|
Year |
2011 |
2012 |
||
|
Plant parts |
MSL |
S. Br |
MSL |
S. Br |
|
July |
1.3 |
01.0 |
1.3 |
00.8 |
|
August |
3.5 |
20.8 |
4.0 |
13.5 |
|
September |
7.0 |
13.5 |
4.3 |
15.5 |
|
October |
1.8 |
02.3 |
1.3 |
08.0 |
Distribution of eggs on plant structures
Preferred nodes on the main stem for oviposition
The female moth preferred the upper ten S. Br for egg-laying. The number of eggs laid were varied significantly from Ter. Bud to the S10 (in 2011:F (10, 151) = 4.03; P-value=0.0001 and in 2012: F (10, 151) = 4.03; P- value=0.0001). Amongst these branches, the most preferred was S1 (just below the top) where most (30-40 eggs) eggs were laid. On the rest of the branches, i.e. Ter. Bud and S1 to the S8, oviposition ranged from 25 to 10 eggs (Fig. 2).
Preferred parts of the plants for oviposition
Similarly, the number of eggs on leaves at various nodes of the main stem starting from the top part downwards (F (7, 112) =2.037; P-value= 0.046 in 2011; F (7, 112) =2.87; P-value=0.0085 in 2012) also varied significantly and eggs were not found below the leaf of sixth node (Fig. 3). Eggs on plant structures likewise Ter. bud, NE leaves, leave, squares, flowers and bolls were varied significantly (2011: F (5, 87) = 8.84: P-value= 0.00 and 2012: F (5, 87) = 3.33, P-value = 0.0085). The most preferred part of the plant for egg laying were as in descending order; leaves, Ter. bud, NE leaves, squares, flowers and bolls (Fig .4).
Dispersion pattern of E. vittella eggs in the field
Taylor’s power law regression showed significantly positive relationships between the log (variance) and log (mean) of eggs of E. vittella per plant in both years. The intercept “a” of Taylor’s power law was <1, and the distribution coefficient “b”>1, indicating an aggregate spatial pattern distribution of eggs in both years (Table III). In contrast, the values obtained from the variance-to-mean ratio (ID) for testing distribution pattern of eggs in cotton field under unsprayed condition were <1 (ranging from 0.38 to 0.55) in each of the 2 years and were combined. Also, d, the chi-square test index, for all the data set was < -1.96 (-3.31, -2.94 and -3.42) and also Lloyd index of patchiness (IP) was < 1 (0.50, 0.29 and 0.30) suggesting that distribution was uniform (Table IV).
Table III.- Parameters for spatial distribution of E. vittella eggs derived from Taylor’s power law with linear regression method.
|
Year |
a |
b |
R2 Adj |
Distribution pattern |
|
2011 |
0.98 ± 0.12;P= 0.00 |
1.83± 0.39; P= 0.0003 |
0.68 |
Aggregate |
|
2012 |
0.92 ± 0.12;P= 0.00 |
1.80± 0.44; P=0.0011 |
0.51 |
Aggregate |
|
2011 and 2012 |
0.90 ± 0.12; P= 0.00 |
1.98± 0.5; P= 0.0014 |
0.50 |
Aggregate |
a, the intercept is a scaling factor; b, the slope for measure of aggregation.
Table IV.- Mean, variance, Chi-square and indices for E. vittella eggs distribution in field conditions.
|
Year |
Mean ± SE |
Variance |
DF |
P-value |
Chi-Square (χ2) |
ID < 1 uniform |
d <−1.96 uniform |
IP < 1 uniform |
|
2011 |
0.91 ±0.18 |
0.50 |
11 |
0.9997 |
1.43 |
0.55 |
-3.31 |
0.50 |
|
2012 |
0.86 ±0.15 |
0.34 |
11 |
0.9980 |
2.13 |
0.39 |
-2.94 |
0.29 |
|
2011 and 2012 |
0.88 ±0.15 |
0.34 |
12 |
1.0000 |
0.56 |
0.38 |
-3.42 |
0.30 |
Discussion
Egg distribution of E. vittella in cotton field
E. vittella egg deposition began in the month of July about 45 days after cotton sowing. It was observed that this pest shifted to cotton from neighboring okra fields in July and remained active in cotton till the crop was harvested in October-November, with the peak pest populations during August-September. This observation was similar to the findings of Solangi et al. (2016) and Syed et al. (2011).
Mechanism of plant selection by E. vittella in the field
In the present study, E. vittella eggs distribution was studied in relation to selection of suitable host cotton plants in the natural field conditions. Out of 225 plants observed, E. vittella eggs were found on only 36-41%, plants with infested MSL on 15-15.6% and S. Br on 36-41% plants. Most of the plants were without damage by E. vittella, except a few plants, which were previously damaged in old bolls (visual observation). More eggs were found on the plants sampled from the central part of a plot. This oviposition behavior indicates that E. vittella female moths has the ability to discriminate between a good and a bad or an unsuitable host plant as it directly concerns to the survival of the offspring (Renwick and Chew, 1994; Awmack and Leather, 2002; Binyameen et al., 2013). Like other lepidopteran pests, E. vittella foraged to select suitable areas and hosts to avoid the risk of predation and/or parasitism (Ramaswamy et al., 1987; Tamhankar et al., 1993), and competition (Tingle and Mitchell, 1992; Hartlieb and Rembold, 1996; Suverkropp et al., 2008; Zakir et al., 2013).
Distribution of E. vittella eggs on plant
Significantly more eggs on S. Br as compared to MSL shows the preference of female moth for outer border of the plants for oviposition. In the current study, the terminal buds and leaves on the main stem down to the sixth node, were preferred as oviposition sites by E. vittella females (Lundgren and Fergen, 2006). Further on the sympodial branches on the main stem starting from 1st node going downward, the first ten branches were likely selected by E. vittella females with the first eight branches more preferred for egg deposition. In addition, the branches were more desirable sites for oviposition than leaves on the main stem. These results revealed that like other lepidopteran pests (Zalom et al., 1983), E. vittella prefer upper plant canopy up to the tenth node and also exterior margin (branches) of the cotton plants. It was also reported earlier by Chakravarthy (1985) that the E. vittella female moths most preferred the top 50 cm of the plant canopy for oviposition. This reflects the hypothesis “preference performance”, presented by Heisswolf et al. (2005) to enhance the performance of E. vittella offspring.
Distribution pattern of E. vittella eggs in the cotton fields
Association between host plants selection and eggs deposition in the cotton determined with SRC reveals significantly positive and random correlation. A similar study was conducted by Pai (2016) and determined various associations between honey bees and different flora. Taylor’s power law of dispersion determines weak correlation with the slope “b”>1, reveals clumbed or aggregated eggs on the plant in the cotton field. Sardana and Tewari (1990) also observed clumped distribution of E. vittella eggs in okra fields using the statistics of negative binomial distribution and the variance to mean ratio, k and chi-square values. Among the other indices, index of dispersion, chi-square test index and Lloyd index of patchiness (ID < 1, d< −1.96 and IP< 1, respectively) indicated that eggs were distributed uniformly in the cotton field. Chakravarthy (1985) noted that the eggs and larvae of Earias spp. followed a uniform distribution on cotton varieties. However, dispersion of Earias eggs on cotton varieties was due to environmental heterogeneity. Very few (5 to 15%) lepidopteran can lay eggs in large clusters; most Lepidoptera lay single eggs and develop as solitary larvae (Lacey and Kaya, 2007). Taylor (1984) and Southwood and Henderson (2009) stated that change in distribution from clumped/aggregated to random or regular resulted from the alteration of the size of the area occupied by the insects related to that of the sample or decreased and continuous population density, the dispersion was effectively random. Nachapong (1980) previously stated that the distribution of cotton bollworm was dependent on both egg laying habits of the female moths and variation in growth among the cotton plants.
Present result further implies site preference for egg laying within plant based on fresh fruiting parts (Sharma and Agarwal, 1983; Dhillon and Sharma, 2004) depending upon female choice (Heisswolf et al., 2005). It was observed that E. vittella female moths equally preferred terminal bud, unexpanded (tender terminal) leaves, fresh leaves, squares, flowers and bolls for sites of oviposition. There was slight variation in the number of eggs deposited on these structures as more eggs were laid on leaves (tender as well as fresh leaves) followed by squares, bolls and flowers. However, Sharma and Agarwal (1983) found E. vittella eggs deposited on the leaves, squares, and Dhillon and Sharma (2004) recorded ovipositions on buds and bolls.
Conclusions
The knowledge of distribution pattern and site selection for egg-laying are basic requirements to develop a management strategy, such as pest monitoring (Ross and Ostlie, 1990) and applications of control measures (Spangler and Calvin, 2001). The current study provides guidelines for both short and long term management of the seasonal outbreaks and detection of E. vittella initial infestation, respectively as an integral part in the IPM strategies. For instance, upper canopy of ten nodes is determined as the most important area of the plants for control measures of E. vittella at egg laying stages through augmentative releases of egg parasitoids such as Trichogramma chilonis or by ovicidal application. E. vittella females prefer the upper canopy of the cotton plants for oviposition as it contains all different types of fruiting bodies ranging from squares to mature green bolls as well as leaves. Moreover, the results from the current study indicate that regular pest scouting should be done after the plant starts its reproductive stage.
Acknowledgements
We thank Dr. William Walker III from Swedish University of Agricultural Sciences for his valuable comments on an earlier version of the manuscript. We are also thankful for financial support of Director of Central Cotton Research Institute, Multan. Head, Mr. Muhammad Rafiq and Chairman Department of Entomology CCRI, Dr. Muhammad Razaq from BZU Multan provided Technical support. The field staff, Mr. Tanveer Hussain, Zahoor Ahmad, and other technical staff members at Entomology-section, CCRI Multan who helped in data collection with minimum human error.
Statement of conflict of interest
The authors have declared no conflict of interests.
References
Arbab, A. and McNeill, M.R., 2014. Spatial distribution and sequential sampling plans for adult Sitona humeralis Stephens (Coleoptera: Curculionidae) in alfalfa. J. Asia-Pacific Ent., 17: 515-519. https://doi.org/10.1016/j.aspen.2014.04.009
Awmack, C.S. and Leather, S.R., 2002. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Ent., 47: 817-844. https://doi.org/10.1146/annurev.ento.47.091201.145300
Aziz, M.A., 2010. Sustainable management of spotted bollworms, Earias vittella (Fabricius) and Earias insulana (Boisduval),(Lepidoptera: Noctuidae) on Okra, Abelmoschus esculentus (L.) in Punjab, Pakistan, Doctoral dissertation. Department of Agric. Entomology, University of Agriculture, Faisalabad.
Aziz, M.A., Hasan, M.U. Ali, A. and Iqbal, J., 2012. Comparative efficacy of different strategies for management of spotted bollworms, Earias spp. on Okra, Abelmoschus esculentus (L.) Moench. Pakistan J. Zool., 44: 1203-1208.
Badiyala, A., 2013. Integrated pest management in OKRA Abelmoschus esculentus L. Moench. http://krishikosh.egranth.ac.in.
Batáry, P., Örvössy, N., Kőrösi, Á. and Peregovits, L., 2008. Egg distribution of the southern festoon (Zerynthia polyxena) (Lepidoptera, Papilionidae). Acta Zool. Acad. Sci. Hungar., 54: 401-410.
Binyameen, M., Hussain, A., Yousefi, F., Birgersson, G. and Schlyter, F., 2013. Modulation of reproductive behaviors by non-host volatiles in the polyphagous Egyptian cotton leafworm, Spodoptera littoralis. J. chem. Ecol., 39: 1273-1283. https://doi.org/10.1007/s10886-013-0354-4
Chakravarthy, A.K., 1985. Distribution patterns, sample-size and sampling Earias spp. on cotton in Ludhiana (Punjab). Int. J. Trop. Insect Sci., 6: 517-520. https://doi.org/10.1017/S1742758400004343
Debouzie, D. and Thioulouse, J., 1986. Statistics to find spatial and temporal structures in populations. In: Pest control: Operations and systems analysis in fruit fly management. Springer, pp. 263-282. https://doi.org/10.1007/978-3-642-70883-1_18
Dhawan, A., Simwat, G. and Sidhu, A., 1990. Shedding of fruiting bodies by bollworms in Asiatic cottons. J. Res. Punjab Agric. Univ., 27: 441-443.
Dhillon, M.K. and Sharma, P.D., 2004. Studies on biology and behavior of Earias vittella (Lepidoptera: Noctuidae) for mechanisms of resistance in different cotton genotypes. Crop Protect., 23: 235-241. https://doi.org/10.1016/j.cropro.2003.08.012
Drake, V.A., 1991. Methods for studying adult movement in Heliothis. In: Heliothis: Research methods and prospects, pp. 109-121. https://doi.org/10.1007/978-1-4612-3016-8_10
Duffield, S.J. and Chapple, D.G., 2001. Within-plant distribution of Helicoverpa armigera (Hübner) and Helicoverpa punctigera (Wallengren) (Lepidoptera: Noctuidae) eggs on irrigated soybean. Aust. J. Ent., 40: 151-157. https://doi.org/10.1046/j.1440-6055.2001.00213.x
Elliott, J.M., 2003. A comparative study of the dispersal of 10 species of stream invertebrates. Freshw. Biol., 48: 1652-1668. https://doi.org/10.1046/j.1365-2427.2003.01117.x
Fauvergue, X., Hopper, K.R. and Montpeier, F., 1994. Spatial distribution of Diuraphis noxia and one of its parasitoids, Diareretiella rapae. Proceedings of the Sixth Russian Wheat Aphids Workshop.
Fernandes, G., Degrande, M.P.E., Cubas, A.C. and Silva, A.M., 2007. Vertical distribution, population density, and natural egg parasitism of cotton leafworm on cotton under IPM. Rev. Colomb. Ent., 33: 27-30.
Gomez, K.A. and Gomez, A.A., 1984. Statistical procedures for agricultural research. John Wiley & Sons.
Hartlieb, E. and Rembold, H., 1996. Behavioral response of female Helicoverpa (Heliothis) armigera (Lepidoptera: Noctuidae) moths to synthetic pigeon pea (Cajanus cajan L.) kairomone. J. chem. Ecol., 22: 821-837. https://doi.org/10.1007/BF02033589
Hasan, W. and Ansari, M.S., 2010. Evaluation of some insecticides against spotted bollworm, Earias vittella (Fab.) on different okra cultivars. Trends Biosci., 3: 41-44.
Heisswolf, A., Obermaier, E. and Poethke, H.J., 2005. Selection of large host plants for oviposition by a monophagous leaf beetle: Nutritional quality or enemy-free space? Ecol. Ent., 30: 299-306. https://doi.org/10.1111/j.0307-6946.2005.00706.x
Holland, J.N., Buchanan, A.L. and Loubeau, R., 2004. Oviposition choice and larval survival of an obligately pollinating granivorous moth. Evol. Ecol. Res., 6: 607-618.
Ifoulis, A.A. and Savopoulou-Soultani, M., 2006. Developing optimum sample size and multistage sampling plans for Lobesia botrana (Lepidoptera: Tortricidae) larval infestation and injury in northern Greece. J. econ. Ent., 99: 1890-1898. https://doi.org/10.1093/jee/99.5.1890
Khaing, O., Hormchan, P., Jamornmarn, S. and Wongpiyasatid, A., 2002. Spatial dispersion and optimum sample size for cotton bollworm, Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) larvae on cotton. Kasetsart J., 36: 235-241.
Kuno, E., 1991. Sampling and analysis of insect populations. Annu. Rev. Ent., 36: 285-304. https://doi.org/10.1146/annurev.en.36.010191.001441
Lacey, L.A. and Kaya, H.K., 2007. Field manual of techniques in invertebrate pathology. Springer. https://doi.org/10.1007/978-1-4020-5933-9
Lloyd, M., 1967. Mean crowding. J. Anim. Ecol., 36: 1-30. https://doi.org/10.2307/3012
Lundgren, J.G. and Fergen, J.K., 2006. The oviposition behavior of the predator Orius insidiosus: Acceptability and preference for different plants. BioControl, 51: 217-227. https://doi.org/10.1007/s10526-005-0609-2
Mallampalli, N. and Isaacs, R., 2002. Distribution of egg and larval populations of cranberry fruitworm (Lepidoptera: Pyralidae) and cherry fruitworm (Lepidoptera: Tortricidae) in highbush blueberries. Environ. Ent., 31: 852-858. https://doi.org/10.1603/0046-225X-31.5.852
STATISTIX, 2008. Statistix 8.1 analytical software. McGraw-Hill, Co., Tallahassee, Florida.
Myers, J.H., 1978. Selecting a measure of dispersion. Environ. Ent., 7: 619-621. https://doi.org/10.1093/ee/7.5.619
Nachapong, M., 1980. Preliminary investigation into the spatial pattern of distribution of Helicoverpa (Heliothis) armigera eggs on cotton. Thai J. Agric. Sci., 13: 343-351.
Pai, A., 2016. A preliminary look at the bee and syrphid diversity in kitchen gardens. Dept. Biology, St. Lawrence University, St. Lawrence County, NY.
Rahman, M.A., Uddin, M.M., Haque, M.A. and Rahman, M.M., 2015. Varietal preference of okra shoot and fruit borer, Earias vittella (Fab.) under field condition in Bangladesh. Acad. Res. J. Agric. Sci. Res., 3: 8-12.
Ramaswamy, S.B., Ma, W.K. and Baker, G.T., 1987. Sensory cues and receptors for oviposition by Heliothis virescens. Ent. Exp. Appl., 43: 159-168. https://doi.org/10.1111/j.1570-7458.1987.tb03600.x
Renwick, J.A.A. and Chew, F.S., 1994. Oviposition behavior in Lepidoptera. Annu. Rev. Ent., 39: 377-400. https://doi.org/10.1146/annurev.en.39.010194.002113
Ross, S.E. and Ostlie, K.R., 1990. Dispersal and survival of early instars of European corn borer (Lepidoptera: Pyralidae) in field corn. J. econ. Ent., 83: 831-836. https://doi.org/10.1093/jee/83.3.831
Sardana, H.R. and Tewari, G.C., 1990. Spatial distribution pattern of eggs of Earias vittella Fabricius in okra field. Entomon, 15: 53-58.
Sharma, H.C. and Agarwal, R.A., 1983. Oviposition behaviour of spotted bollworm, Earias vittella Fab. on some cotton genotypes. Int. J. Trop. Insect Sci., 4: 373-376. https://doi.org/10.1017/S1742758400002411
Sidhu, A.S. and Sandhu, S.S., 1977. Damage due to the spotted bollworm (Earias vittella Fabr.) in relation to the age of bolls of hirsutum variety j-34. J. Res. Punjab Agric. Univ., 14: 184-187.
Solangi, B.K., Suthar, V., Rustamani, M.A., Abro, N.A., Abbasi, A.R. and Abro, M.N., 2016. Comparative bollworm infestation on bt and non-bt cotton. Sindh Univ. Res. J. (Sci. Ser.), 48: 23-28.
Southwood, T.R.E. and Henderson, P.A., 2009. Ecological methods. John Wiley & Sons.
Spangler, S.M. and Calvin, D.D., 2001. Vertical distribution of European corn borer (Lepidoptera: Crambidae) egg masses on sweet corn. Environ. Ent., 30: 274-279. https://doi.org/10.1603/0046-225X-30.2.274
Suverkropp, B.P., Dutton, A., Bigler, F. and van Lenteren, J.C., 2008. Oviposition behaviour and egg distribution of the European corn borer, Ostrinia nubilalis, on maize, and its effect on host finding by Trichogramma egg parasitoids. Bull. Insectol., 61: 303-312.
Syed, T.S., Abro, G.H., Khanum, A. and Sattar, M., 2011. Effect of host plants on the biology of Earias vittella (Fab) (Noctuidae: Lepidoptera) under laboratory conditions. Pakistan J. Zool., 43: 127-132.
Tamhankar, A.J., Gothi, K.K. and Rahalkar, G.W., 1993. Host-induced augmented reproduction in spotted bollworm, Earias vittella (Fabricius) (Lepidoptera: Noctuidae). Int. J. Trop. Insect Sci., 14: 371-375. https://doi.org/10.1017/S1742758400014880
Taylor, L.R., 1984. Assessing and interpreting the spatial distributions of insect populations. Annu. Rev. Ent., 29: 321-357. https://doi.org/10.1146/annurev.en.29.010184.001541
Tingle, F.C., and Mitchell, E.R., 1992. Attraction of Heliothis virescens (F.) (Lepidoptera: Noctuidae) to volatiles from extracts of cotton flowers. J. chem. Ecol., 18: 907-914. https://doi.org/10.1007/BF00988331
Torres, J.B., Faria, C.A., Evangelista, Jr. W.S. and Pratissoli, D., 2001. Within-plant distribution of the leaf miner Tuta absoluta (Meyrick) immatures in processing tomatoes, with notes on plant phenology. Int. J. Pest Manage., 47: 173-178. https://doi.org/10.1080/02670870010011091
Vennila, S., Biradar, V.K., Panchbhai, P.R., Gadpayle, J.G., Deshmukh, A.Y., Nemade, P.W. and Karanjkar, P.P., 2005. Seasonal dynamics, survival and feeding preference of Earias vittella (Fab.) larval instars on cotton. Annls Pl. Protec. Sci., 13: 60-64.
Wilson, L.T., Gutierrez, A.P. and Leigh, T.F., 1980. Within-plant distribution of the immatures of Heliothis zea (Boddie) on cotton. Hilgardia, 48: 12-23. https://doi.org/10.3733/hilg.v48n02p012
Zakir, A., Sadek, M.M., Bengtsson, M., Hansson, B.S., Witzgall, P. and Anderson, P., 2013. Herbivore-induced plant volatiles provide associational resistance against an ovipositing herbivore. J. Ecol., 101: 410-417. https://doi.org/10.1111/1365-2745.12041
Zalom, F.G., Wilson, L.T. and Smith, R., 1983. Oviposition patterns by several lepidopterous pests on processing tomatoes in California. Environ. Ent., 12: 1133-1137. https://doi.org/10.1093/ee/12.4.1133
To share on other social networks, click on any share button. What are these?









