Comparative Toxicity of Insecticides against Trichogramma chilonis (Hymenoptera; Trichogrammatidae) under Laboratory Conditions
Research Article
Comparative Toxicity of Insecticides against Trichogramma chilonis (Hymenoptera; Trichogrammatidae) under Laboratory Conditions
Kanwal Hanif1, Dilbar Hussain1, Qurban Ali1, Asad Aslam1*, Tamsila Nazir1, Muhammad Saleem1, Muhammad Faheem Akhtar1, Muhammad Zubair2, Najuf Awais Anjum3 and Muhammad Umar Qasim1
1Entomological Research Institute, AARI, Faisalabad, Pakistan; 2Oilseeds Research Institute Faisalabad, AARI, Pakistan; 3Entomological Research Sub-station, Pasrur, Pakistan.
Abstract | Trichogramma (Hymenoptera; Trichogrammatidae) is a genus of parasitic wasps that are powerful biocontrol agents against a variety of insect pests. These biological control agents retain their importance due to their easy mass rearing, high searching ability and effectiveness against many crop insect pests. In this study the toxicological effect of seven insecticides viz Flubendiamide (Belt 480 SC)@ 500 ul/l, Pyriproxyfen (Priority 10.8 EC) @ 2000 µl/l, Chlorantraniliprole + Thiamethoxam (Voliam Flexi 300 SC) @ 800µl/l, Nitenpyram (Pyramid 10% AS) @ 2000 µl/l, Lufenuron (Match)@ 2000 µl/l, Chlorantraniliprole (Coragen 20% SC) @ 200 µl/l and Flonicamid (Ulala50% WG) @ 60 mg/l were evaluated on the viability of parasitized eggs, survival and parasitism efficacy of Trichogramma chilonis under controlled conditions at toxicology laboratory Entomological Research Institute Ayub Agriculture Research Institute, Faisalabad. The viability of eggs was evaluated by exposing egg cards to insecticides by dipping them in insecticide solution for 10 seconds while adult survival was investigated through dipped surface residue bioassay method. The results revealed that new chemistry insecticides viz., Flubendiamide, Flonicamid and Chlorantraniliprole were comparatively safer to egg parasitiod followed by Chlorantraniliprole while Nitenpyram and Pyriproxyfen was found toxic to Trichogramma. Flubendiamide, Chlorantraniliprole and Flonicamidwas proved comparatively less toxic against T. chilonis with emergence percentage while in the case of survival of T. chiloniswasps, the results revealed that Pyriproxyfen and Nitenpyram had less knockdown effect as compared to other insecticides while Lufenuron was most toxic at 4 h post treatment and at 24 h all the insecticides were equally toxic to the parasitoid at adult stage.
Received | April 25, 2022; Accepted | June 10, 2022; Published | October 15, 2022
*Correspondence | Asad Aslam, Entomological Research Institute, AARI, Faisalabad, Pakistan; Email: mr.awan2233@gmail.com
Citation | Hanif, K., D. Hussain, Q. Ali, A. Aslam, T. Nazir, M. Saleem, M.F. Akhtar, M. Zubair, N.A. Anjum and M.U. Qasim. 2022. Comparative toxicity of insecticides against Trichogramma chilonis (Hymenoptera; Trichogrammatidae) under laboratory conditions. Journal of Innovative Sciences, 8(2): 195-201.
DOI | https://dx.doi.org/10.17582/journal.jis/2022/8.2.195.201
Keywords | Biological control, Egg parasitoid, Insecticides, Trichogramma chilonis, Comparative toxicology
Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
1. Introduction
Trichogrammatids are tiny endoparasite wasps, 0.2-1.5 mm in size. They belong to common group of egg parasitoids and used for biological control (Chailleux et al., 2012, 2013; Tabone et al., 2010) as an important tool in the integrated pest management. The species of Trichogrammatids are extensively used natural enemies insect in the world as they are easy to mass rare and attack many insect pests of important crops (Sarwar and Salman, 2015; Parra et al., 2010). They have the capacity to increase in number rapidly and are very destructive parasitoids of insect eggs especially eggs of butterflies and moths. They sometimes show 100% parasitism during optimum environmental conditions periods and the increasing parasitizing females showed the optimum temperature range 25-30°C (Nadeem et al., 2009; Reznik et al., 2009). It has been shown that two species i.e. T. nubilale Ertle and Davis are mostly active for controlling corn pests of Lepidoptera order (Wang et al., 1999). T. chilonis attacks on the lepidopterans of more than 400 pest species. It has been effectively used in the inoculative and inundative biocontrol programme around the world (Wang et al., 1999).
The genus Trichogramma is very effective taxa in studies of biological control programs. Members of Trichogramma genus are among one of the 80 genera in the Trichogrammatidae family and act as eggparasitoids of insects. The species of Trichogramma which are mostly collected from orchard and crops are T. brevicapillum, T. thalense, T. atopovirilia, T. pretiosum and T. chilonis, T. platneri, T. deion, T. nubilale, T. exiguum, T. minutum and T. fuentesi. The adult female wasps laid eggs into the eggs of moths. But few species of the Trichogramma parasitize also the eggs of beetles (Coleoptera), flies (Diptera), other wasps (Hymenoptera), true bugs (Heteropteran), lacewings and their relatives (Neuropteran) (Sarwar and Salman, 2015). Species of Trichogramma are mostly used against insect pests of different crops as biological control agent through release and augmentation. Trichogramma species saves more than 16 million ha crops by destroying the pests through parasitization (Singhamuni et al., 2015).
In integrated pest management program, use of insecticide remains a major control technique because it is cost-effective, efficient and easy for applying. However, use of chemicals along with reducing the number of target pests, affects the action of beneficial insects too (Wang et al., 2018; Umar et al., 2021; Saleem et al., 2021). Chemical control with Trichogramma have been measured incompatible because of the negative impact of insecticides. Toxic insecticides and Trichogramma are carefully planned in the same crop and these are mostly incompatible (Saber, 2011). Insecticides are used in many countries for the control of insect pests of soybean, vegetables, pine, rice, corn, sugarcane, cotton and sugar beet (Saleem el al., 2021). The natural population of T. chilonis contributed for the control of pests’ population. Therefore, the effects of environmental pollutions on this specie by the insecticides also showed significant risk for the protection of biodiversity (Delpuech et al., 1998; Shehzad et al., 2021). T. chilonis are mostly exposed in the foraging process to insecticides in the lepidopterous pests of IPM program. Studies on the harmful effects of pesticides on Trichogramma have been inadequate (Lingathurai et al., 2015).
The females of T. chilonis discriminate the parasitized host eggs from unparasitized ones, they are mostly parasitized by heterospecific females (Trichogrammabactrae Nagaraja). They focus on lying of eggs and stay in the host eggs that are unparasitize. On the of insecticides no information is available in T. chilonis on their host discernment behavior (Wang et al., 2016). Pesticide resistance, severe pesticide regulation, secondary pest outbreak and concern about environment quality and human health have extended the awareness in IPM that emphasizes biocontrol and minimizing the use of insecticides. Current research was conducted to evaluate the toxic effect of seven insecticides viz., Flubendiamide, Pyriproxyfen, Chlorantraniliprole + Thiamethoxam, Nitenpyram, Lufenuron, Chlorantraniliprole and Flonicamid against immature and adult stages of T. chilonis after four and twenty-four hour of exposure to the insecticides and compatibility of the insecticides with this biological control agent.
2. Materials and Methods
The experiment was carried out at the toxicology laboratory of Entomological Research Institute, AARI, Faisalabad under controlled conditions to evaluate the toxic effect of different insecticides on the viability of parasitized eggs, survival and parasitism efficacy of T. chilonis by using different bioassay methods.
2.1 Insecticides
Seven insecticides viz., Flubendiamide (Belt 480 SC) @ 50 ml/acre, Pyriproxyfen (Priority 10.8 EC) @ 500ml/acre, Chlorantraniliprole + Thiamethoxam (Voliam Flexi 300 SC) @ 80 ml/acre, Nitenpyram (Pyramid 10% AS) @ 200ml/acre, Lufenuron (Match)@ 200ml/acre Chlorantraniliprole (Coragen 20% SC) @ 80ml/acre and Flonicamid (Ulala50% WG) @ 60g/acre were evaluated against T. chilonisat adult and egg stages. Lab doses were calculated through field recommended doses using Abbot’s formula (given below). Each treatment was replicated three times under CRD design. The concentrations of insecticides were measured with the help of micro pipette and properly mixed with 1 liter distilled water in a beaker (1000 ml) to make solutions. For control treatment, only distilled water was used. Following equation was used to prepare the required solution (Abbott, 1925).
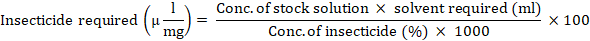
2.2 Egg card bioassay
The card was directly exposed to the five treatments in beakers to evaluate the viability of eggs and their efficacy of parasitism. The bioassay was conducted on the 1st, 2nd, 3rd, 4th, 5th, 6th, and 7th day old parasitized Sitotroga cerealella eggs. Five randomly selected cards containing 40 parasitized eggs were dipped into each treatment for 10 seconds. After that, the dipped egg cards were dried in an ambient room. Each treated egg card was placed in a small glass petri dish (5 cm diameter and 0.5 cm deep) until healthy parasitic wasp was emerged. Number of emerged wasps were then counted for percent emergence.
2.3 Dipped surface residue bioassay
The effect of insecticide residues on the survival of adult T. chilonis was investigated by using a dipped surface residue bioassay. The experiment was carried out in ventilated glass bio-assay chambers (15 cm x 4 cm). The whatman filter paper (25 mm) was dipped into the each treatment, then dried and placed in a glass bioassay tube. Twenty adults of T. chilonis were released in each tube. After 4 hours and 24 hours of exposure to treatments, the number of alive and dead wasps was counted. Each treatment was replicated 3 times.
2.4 Data analysis
The following formula was used to determine the percentage of wasps emerged.
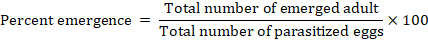
While in case of percentage mortality was calculated by using following formula.
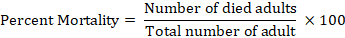
Then the mean of variance was statistically analysis by using Statistix 8.1 software.
Table 1: Percentage egg viability and Percent emergence of Trichogramma chilonis.
|
Treatments |
1-day old egg parasitoid Mean±SE |
2 days old egg parasitoid Mean±SE |
3 days old egg parasitoid Mean±SE |
4 days old egg parasitoid Mean±SE |
5 days old egg parasitoid Mean±SE |
6 days sold egg parasitoid Mean±SE |
7 days old egg parasitoid Mean±SE |
|
Control |
37.80± 0.52a |
23.40+ 0.40a |
32.00± 0.30a |
35.60± 0.79a |
29.20± 0.94a |
24.60± 0.72a |
20.80± 0.75a |
|
Flubendiamide (Belt 480 SC) @ 500 ul/l |
17.80± 0.91c |
17.80+ 0.91a |
21.00± 1.18b |
20.80± 0.63b |
26.20± 0.37a |
12.00± 0.67c |
0.40±0.25c |
|
Pyriproxyfen (Priority 10.8 EC) @ 2000 µl/l |
10.60± 1.21ef |
6.40 +0.41cd |
16.80± 0.98bc |
4.80± 0.31d |
1.20± 0.31d |
13.40± 0.86bc |
0.60± 0.05c |
|
Chlorant-raniliprole +Thiame-thoxam (Voliam Flexi 300 SC) @ 800 µl/l |
10.20± 1.10f |
5.00+ 0.38d |
4.00± 0.86e |
3.40± 0.42e |
2.20± 0.41d |
15.00± 0.31ab |
3.80± 0.17b |
|
Nitenpyram (Pyramid 10% AS) @ 2000 µl/l |
15.80± 0.95c |
11.20± 0.73bc |
11.80± 0.44cd |
9.00± 0.86c |
23.80± 1.17c |
13.80± 0.53bc |
0.20± 0.09d |
|
Lufenuron (Match) @ 2000 µl/l |
12.80± 0.97de |
8.00± 0.62cd |
14.20± 0.79d |
29.00± 1.50a |
13.80± 0.35bc |
12.20± 0.71c |
0.00± 0.04e |
|
Chlorantr-aniliprole (Coragen 20% SC) @ 200 µl/l |
13.40± 0.50b |
16.40± 1.15b |
23.00± 0.60b |
25.40± 0.87ab |
17.40± 0.67bc |
13.20± 0.89bc |
0.00±0.04e |
|
Flonicamid (Ulala 50% WG) @ 60 mg/l |
20.80± 0.61b |
7.40± 0.52cd |
8.40± 0.56e |
2.20± 0.40e |
24.80± 0.42ab |
16.00± 0.61a |
0.40±0.15d |
3. Results and Discussion
3.1 Percent emergence of Trichogramma chilonis
Exposure of Lufenuron and Chlorantraniliprole (Coragen 20% SC) to parasitoid, T. chilonis, Showed lowest emergence (0.00% to 8.00% and 0.00% to 13.20%) respectively whereas, the highest mean emergence, when no treatment was used (control) was observed as 20.80±0.75a (56%) to 37.80±0.52a (96%) was observed. The emergence in 5 days old parasitoid eggs after the treatment of Chlorantraniliprole+Thiamethoxamwas@ 800 µl/l was found as 2.20±0.41d (6%)(P=0.00; F=12.9) and for Flubendiamide@ 500 ul/l, Pyriproxyfen@ 2000 µl/l, Nitenpyram@ 2000 µl/l, Lufenuron@ 2000 µl/l, Chlorantraniliprole@ 200 µl/land Flonicamid 60 mg/l it was found as 0.40±0.25c (1%) 0.60±0.05c (2%), 0.20±0.09d (1%), 0.00±0.04e (0%), 0.00±0.04e (0%)and 0.40±0.15d (1%)(P=0.0068, F=3.49) in 7 days old eggs that was proved to have lowest effect on the T. chilonis emergence and showed harmless to the egg parasitoid. New Chemistry insecticides viz., Flubendiamide, Flonicamid and Chlorantraniliprole observed to be most safe to egg parasitiod while Chlorantraniliprole+Thiamethoxam and Lufenuron found safer as compared to Nitenpyram and Pyriproxyfen.
3.2 Survival rate
In this study, all the insecticides differed significantly regarding survival of adults of T. chilonis 4 hours post application (Table 2). Pyriproxyfen resulted in maximum survival72.34 % of adults of egg parasitoid after 4 hour of treatments application followed by Nitenpyram (68.22% survival) which was found least toxic insecticides. On other hand Lufenuron was found most toxic insecticide resulted in lowest survival (27.44%) of the adult parasitoids which was significantly different survival percentage rate from Chlorantraniliprole (44.89%) and Flonicamid (41.55%).
Results after 24 hours of treatments application revealed that Flubendiamide (8.37%) was least toxic to the survival of T. chilonis adults followed by Chlorantraniliprole + Thiamethoxam (4.03%) and Nitenpyram (4.03%) while Nitenpyram, Lufenuron and Flonicamid showed 100% moratlity percentage so all insecticdes were found toxic to the adults, survival percentage ranged as compared to 82.43% in control.
From these results, it is clear that Pyriproxyfen and Nitenpyram had less knockdown effect as compared to other insecticides while Lufenuron was most toxic at 4 h post treatment and at 24 h all the insecticides were equally toxic to the parasitoid at adult stage.
Unsystematic use of pesticides is important aspect which causing the resurgence of insect pest and mortality of natural enemies. The present study indicates that Lufenuron was moderately toxic to T. chilonisand this has already been observed in previous studies of (Sattar et al., 2011). It may be compatible due to a difference in exposure period or dose rate, but the adult Trichogramma’s effect on Lufenuron is likewise very less toxic than the other pesticides after 4 hours. Which are favored by other scientists (Hussainet al., 2012) resulted that Lufenuron applications were safer. In the same way (Anand and Mallapur, 2016) stated it is less toxic. But after 24 hours, 100% mortality was observed and it was found that Lufenuron was not safer for Trichogrammachilonis adult.
Flubendiamide (Belt 480 SC) treatment was moderately toxic. Our findings are quietly in line to that of Sattar et al. (2011) and Hussain et al. (2012). Sattar et al. (2011) resulted in study that Flubendiamide was moderately toxic. Similarly, Hussain et al. (2012) revealed that Flubendiamide was less toxic.
Table 2: Survival (%) of T. chilonis adults at different post treatment intervals of insecticides.
|
Insecticides/Treatments |
Percentage survival |
|
|
After 4 h |
After 24 h |
|
|
Flubendiamide (Belt 480 SC) @ 500 ul/l |
46.44 D |
8.37 B |
|
Pyriproxyfen (Priority 10.8 EC) @ 2000 µl/l |
72.34 B |
0.00 D |
|
Chlorantraniliprole +Thiamethoxam (Voliam Flexi 300 SC) @ 800 µl/l |
46.33 D |
4.03 C |
|
Nitenpyram (Pyramid 10% AS) @ 2000 µl/l |
68.22 C |
4.03 C |
|
Lufenuron (Match) @ 2000 µl/l |
27.44 F |
0.00 D |
|
Chlorantraniliprole (Coragen 20% SC) @ 200 µl/l |
44.89 D |
0.00 D |
|
Flonicamid (Ulala50% WG) @ 60 mg/l |
41.55 E |
0.00 D |
|
Control |
92.77 A |
82.43 A |
Chlorantraniliprole (Coragen 20% SC) was comparatively less toxic after exposure to egg parasitoid T. chilonis. Some other scientists also same resulted that it was less toxic for egg parasitoid (Hussain et al., 2012; Uma et al., 2014).
When T. chilonis adults treated with nitenpyram, it causes knockdown effect and 100% mortality was observed after 24 h of treatments. Our results were in accordance with (Ko et al., 2015) also found 95% mortality and also with (Preetha et al., 2009; Zhao et al., 2012).
Chlorantraniliprole + Thiamethoxam (Voliam Flexi 300 SC) was found moderately toxic in this study. Similarly, (Baehaki et al.,2017) reported that Chlorantraniliprole + thiamethoxam were moderately toxic. All adult behavior after 4 h showed less toxicity whereas, after 24 hours was proved to be highly toxic. However new chemistry insecticides with various modes of action were discovered to be most commonly utilized for their target host and their effect on the host was greater than that of their natural enemies. Therefore, they are rare bio control agents and best fit IPM programme.
As percent survival is concerned, our findings relates to those of Hussain et al. (2010) who stated that at Adult T. chilonis exposed to Imidacloprid, Emamectin benzoate, and Lufenuron had 70.0, 27.6, and 18.4% survival after 3 hours, respectively, however all pesticides were harmful to adult T. chilonis after 24 hours.
Conclusions and Recommendations
Flubendiamide (Belt 480 SC), chlorantraniliprole (Coragen 20% SC) and Flonicamid (Ulala 50% DF) proved comparatively less toxic against T. chilonis with emergence percentage. Chlorantraniliprole + Thiamethoxam (Voliam Flexi 300 SC) and Lufenuron showed moderate toxicity, Pyriproxyfen and Nitenpyram proved highly toxic. All insecticides proved highly toxic against adult stage after twenty-four hours exposure to insecticides and after four hours exposure to insecticides showed less toxicity as per mortality percentage data.
Novelty Statement
The biological control measures are major pillar of integrated pest management (IPM) and broad spectrum insecticides are major thread for the survival and efficacy of parasite so the results of current study will help the judicial l use of insecticides.
Author’s Contribution
QA and DH design supervises the trial. KH, MS and AA execute the trial. TN, MFA, MZ and NAA wrote the research article. MUQ, KH and AA statistical analyzed the data. MZ provided helpful material for experiment.
Statement of conflict of interest
The authors have declared no conflict of interest.
References
Abbott, S.W., 1925. A method of competing the effectiveness of an insecticide. Journal of Economic Entomology, 8: 265-267. https://doi.org/10.1093/jee/18.2.265a
Anand, H., and Mallapur, C.P., 2016. Evaluation of toxicity of insecticides on Chrysoperlazastro wisillemi (Esben-Peterson) and Trichogramma chilonis (Ishii) under laboratory conditions. Journal of Farm Sciences, 29(3): 352-354.
Baehaki, S.E., Surahmat, E.C., Susetyo, A., and Senn, R., 2017. Safety selected insecticides to predators and egg parasitoids of planthoppers in rice ecosystem. American Journal of Engineering Research, 6(6): 174-182.
Chailleux, A., Biondi, A., Han, P., Tabone, E., and Desneux, N., 2013. Suitability of the pest–plant system Tutaabsoluta (Lepidoptera: Gelechiidae) tomato for Trichogramma (Hymenoptera: Trichogrammatidae) parasitoids and insights for biological control. Journal of Economic Entomology, 106(6): 2310-2321. https://doi.org/10.1603/EC13092
Chailleux, A., Desneux, N., Seguret, J., Do ThiKhanh, H., Maignet, P., and Tabone, E., 2012. Assessing European egg parasitoids as a mean of controlling the invasive South American tomato pinworm Tutaabsoluta. PLoS One, 7(10): e48068. https://doi.org/10.1371/journal.pone.0048068
Delpuech, J.M., Froment, B., Fouillet, P., Pompanon, F., Janillon, S., and Boulétreau, M., 1998. Inhibition of sex pheromone communications of Trichogramma brassicae (Hymenoptera) by the insecticide chlorpyrifos. Environ. Toxicol. Chem., 17(6): 1107- 1113. https://doi.org/10.1002/etc.5620170617
Hussain, D., Akram, M., Iqbal, Z., Ali, A., and Saleem, M., 2010. Effect of some insecticides on Trichogramma chilonis(Ishii) (Trichogrammatidae: Hymenoptera) immature and adult survival. Journal of Agricultural Research, 48(4): 531-537.
Hussain, D., Ali, A., Mushtaq-ul-Hassan, M., Ali, S., Saleem, M., and Nadeem, S., 2012. Evaluation of toxicity of some new insecticides against egg parasitoid Trichogramma chilonis (Ishii) (Hymenoptera: Trichogrammitidae). Pakistan Journal of Zoology, 44(4):
Ko, K., Liu, Y., Hou, M., Babendreier, D., Zhang, F. and Song, K., 2015. Toxicity of insecticides targeting rice planthoppers to adult and immature stages of Trichogramma chilonis (Hymenoptera: Trichogrammatidae). Journal of Economic Entomology, 108(1): 69-76. https://doi.org/10.1093/jee/tou053
Lingathurai, S., Pushpalatha, M., Raveen, R., Priyatharsini, P.V., Sathikumaran, R., and Narayanan, P.S., 2015. Ecotoxicological performances and biochemical effect of selected pesticides on Trichogrammachilonis (Ishii). (Hymenoptera: Trichogrammatidae). Journal of Entomology and Zoology Studies, 3(1): 109-114.
Nadeem, F., Kvicera, V., Awan, M.S., Leitgeb, E., Muhammad, S.S., and Kandus, G., 2009. Weather effects on hybrid FSO/RF communication link. IEEE Journal on Selected Areas in Communications, 27(9). https://doi.org/10.1109/JSAC.2009.091218
Preetha, G., Stanley, J., Suresh, S., Kuttalam, S., and Samiyappan, R., 2009. Toxicity of selected insecticides to Trichogramma chilonis: Assessing their safety in the rice ecosystem. Phytoparasitica, 37: 209–215. https://doi.org/10.1007/s12600-009-0031-x
Parra, A., Madrid, R., Echevarria, D., Del Olmo, S., Morenilla-Palao, C., Acosta, M.C., and Belmonte, C., 2010. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nature Medicine, 16(12): 1396. https://doi.org/10.1038/nm.2264
Reznik, D., Lokshin, K., Mitchell, D.C., Parshall, D., Dmowski, W., Lamago, D., and Sales, B.C., 2009. Phonons in doped and undopedBaFe 2 As 2 investigated by inelastic x-ray scattering. Physical Review B, 80(21): 214534. https://doi.org/10.1103/PhysRevB.80.214534
Saber, M., 2011. Acute and population level toxicity of imidacloprid and fenpyroximate on an important egg parasitoid, Trichogramma cacoeciae (Hymenoptera: Trichogrammatidae). Ecotoxicology, 20(6): 1476-1484. https://doi.org/10.1007/s10646-011-0704-3
Saleem, M.J., Hafeez, F., Ali, Q., Aslam, A., Arshad, M., Hussain, D., Iftikhar, A., Naeem, A. and Saleem, M., 2021. Susceptibility of Helicoverpa armigera Hübner (Lepidoptera: Noctuidae) on detached plant parts of transgenic and non-transgenic cotton. International Journal of Tropical Insect Sciences, 42:1239-1244. https://doi.org/10.1007/s42690-021-00642-0
Sarwar, M., and Salman, M., 2015. Toxicity of oils formulation as a new useful tool in crop protection for insect pests control. International Journal of Chemical and Biomolecular Science, 1(4): 297-302.
Sarwar, M., and Salman, M., 2015. Biological insecticide Trichogramma spp. (Hymenoptera: Trichogrammatidae) strikes for caterpillar control. International Journal of Entomology Research, 1(1): 31-36.
Sattar, S., Farmanullah, A., Arif, M., Sattar, H., and Qazi, J.I., 2011. Toxicity of some new insecticides against Trichogrammachilonis (Hymenoptera: Trichogrammatidae) under laboratory and extended laboratory conditions. Pakistan Journal of Zoology, 43: 1117-1125.
Shehzad, M., Tariq, M., Ali, Q., Aslam, A., Mukhtar, T., Akhtar, F.A., Gulzar, A. and Faisal, M., 2021. Evaluation of insecticidal activity of Beauveria bassiana against different instar larvae of Plutella xylostella by using two different methods of application. International Journal of Tropical Insect Sciences, 42:1471-1476. https://doi.org/10.1007/s42690-021-00665-7
Singhamuni, S.A.A., Hemachandra, K.S., and Sirisena, U.G.A.I., 2015. Potential for mass rearing of the egg parasitoids, Trichogrammachilonis and Tricogrammaachaeae (Hymenoptera: Trichogrammatidae) on Corcyra cephalonica eggs. Tropical Agricultural Research, 27(1): 1-12. https://doi.org/10.4038/tar.v27i1.8149
Sudhanan, V.M., Choudhary, R., Bhukaria, A., and Chaudhary, S.S., 2015. Woolly hair with complete atrioventricular dissociation: A rare association. International Journal of Trichology, 7(2): 82. https://doi.org/10.4103/0974-7753.160120
Tabone, E., Bardon, C., Desneux, N., and Wajnberg, E., 2010. Parasitism of different Trichogramma species and strains on Plutellaxylostella L. on greenhouse cauliflower. Journal of Pest Science, 83(3): 251-256. https://doi.org/10.1007/s10340-010-0292-7
Uma, S., Jacob, S., and Lyla, K.R., 2014. Acute contact toxicity of selected conventional and novel insecticides to Trichogramma japonicum Ashmead (Hymenoptera: Trichogrammatidae). Journal of Biopesticides, 7(4), 133.
Umar, M.Y., Zia, S., Ahmad, M., Ali, Q., Anwar, H., Akhtar, M.F., Qasim, M.U. and Aslam, A., 2021. Bio- Wfficacy of some new chemistry insecticides against sugarcane top borer, Scripophaganivella (fabriccius) under field conditions. Journal of Agricultural Research, 59(4): 367-373.
Wang, H., Bloom, O., Zhang, M., Vishnubhakat, J.M., Ombrellino, M., Che, J., and Manogue, K.R., 1999. HMG-1 as a late mediator of endotoxin lethality in mice. Science, 285(5425): 248-251. https://doi.org/10.1126/science.285.5425.248
Wang, D., Lü, L., and He, Y., 2018. Effects of insecticides on sex pheromone communication and mating behavior in Trichogramma chilonis. Journal of Pest Science, 91(1): 65-78. https://doi.org/10.1007/s10340-017-0864-x
Wang, H., Naghavi, M., Allen, C., Barber, R.M., Bhutta, Z.A., Carter, A., and Coggeshall, M., 2016. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the global burden of disease study 2015. The Lancet, 388(10053): 1459-1544.
Zhao, X., Wu, C., Wang, Y., Cang, T., Chen, L., Yu, R., and Wang, A., 2012. Assessment of toxicity risk of insecticides used in rice ecosystem on Trichogramma japonicum, an egg parasitoid of rice lepidopterans. Journal of Economic Entomology, 105: 92–101. https://doi.org/10.1603/EC11259
To share on other social networks, click on any share button. What are these?




