Effect of Different Levels of Soil Moisture and Trichoderma Viride in Controlling Black Scurf of Potato
Research Article
Effect of Different Levels of Soil Moisture and Trichoderma Viride in Controlling Black Scurf of Potato
Azra Nadeem1*, Shaukat Hussain2, Saeedullah2, Asma Akbar3, Zahoor Ahmad4, Maryam Tariq5, Robina Karim6 and Muhammad Huzaifa7
1Department of Plant Pathology, Amir Muhammad Khan Campus Mardan, The University of Agriculture Peshawar, Pakistan; 2Department of Plant Pathology, Faculty of Crop Protection Sciences, The University of Agriculture Peshawar, Pakistan; 3Crop Diseases Research Institute, National Agricultural Research Centre, Islamabad, Pakistan; 4Adaptive Research Program Quetta, Baluchistan, Pakistan; 5Crop Diseases Research Institute, Murree, Pakistan; 6 Department of Agricultural and Applied Economics, Amir Muhammad Khan Campus Mardan, The University of Agriculture Peshawar, Pakistan; 7Department of Horticulture, Faculty of Agriculture, Abdul Wali Khan University Mardan, Pakistan.
Abstract | The interactive effect of 5 different soil moisture levels (3%, 5%, 8%, 11% and 15%) with or without Trichoderma viride as biocontrol agent in controlling black scurf was investigated under controlled conditions. Data were recorded for disease severity, incidence, tuber weight, number of tubers/plant, fresh root weight and plant height at the time of harvest of the crop. The biocontrol agent (T. viride) showed lowest disease incidence (53.73%) and severity (1.27) at 8% moisture level. There was a reduction of 43.33% and 63.29% over control by T. viride for disease incidence and severity respectively at this moisture level. The effect of soil moisture was highly significant and maximum tuber weight was recorded at 15% soil moisture. Both fresh root weight and plant height were not significantly affected by fungal treatments and moisture levels under the conditions of the experiment. The biocontrol agent exhibited good results in terms of disease control and improving yield parameters, between 8 and 15% moisture levels.
Received | March 19, 2021; Accepted | July 17, 2021; Published | November 01, 2021
*Correspondence | Azra Nadeem, Department of Plant Pathology, Amir Muhammad Khan Campus Mardan, The University of Agriculture Peshawar, Pakistan; Email: azranadeem@aup.edu.pk
Citation | Nadeem A., S. Hussain, Saeedullah, A. Akbar, Z. Ahmad, M. Tariq, R. Karim and M. Huzaifa. 2022. Effect of Different Levels of soil moisture and trichoderma Viride in controlling black scurf of potato. Sarhad Journal of Agriculture, 38(1): 40-45.
DOI | https://dx.doi.org/10.17582/journal.sja/2022/38.1.40.45
Keywords | Potato black scurf, Rhizoctonia solani, Disease management, richoderma viride, Soil moisture
Introduction
Potato, known as the king of vegetables, occupies a prominent position among the crops, and has been used as food since long (Kumar et al., 2018). It is a major source of food and has various proportions of carbohydrates, proteins, vitamins, minerals and trace elements (Malik, 1995). However, the composition of these nutrients is affected by a number of factors including cultivar type, temperature, soil moisture and nutrients (Gould, 1999). Diseases also play an important role in limiting potato production. Tuber borne diseases in general and black scurf, caused by Rhizoctonia solani Kuhn in particular has contributed to lower yield over the past so many years.
Scurf is the formation of black sclerotia on newly formed tubers. Other disease symptoms include death of pre-emerging sprouts, cankers on underground stem and stolons, suppressed root system and formation of sclerotia on progeny tubers. Sclerotia not only downgrade tuber quality (Jager et al., 1991) but also result in development of malformed tubers. Since tuber size and number are also affected, yield is considerably reduced. Reduction in quality and yield commonly occurs where cool, moist environment prevails. Conversely, it is less common or may not occur where warmer, drier environmental conditions are the norm (Carling and Leiner, 1990). In view of the importance of this disease and the concurrent losses, need for its management persists.
Rhizoctonia diseases are normally managed by cultural practices, such as crop rotation with grains and methods that minimize prolonged contact of the plant or tubers with the pathogen. These include planting in warm and dry conditions to promote rapid emergence and early harvesting of tubers from the field (Secor and Gudmestad, 1999). Similarly, use of fungicides is often recommended when losses are enormous and when other control measures are not feasible (Rauf et al., 2007). However, cultural and chemical control tactics are admittedly not very effective and the disease remains a persistent problem.
Biological control of diseases has been demonstrated as an additional strategy providing effective and sustainable management in many situations. It can be an effective means of control where other measures are either unavailable or impractical (Tsror et al., 2000). Several microbial antagonists have exhibited potential for control of R. solani in potato (Ristaino and Papavizas, 1985). Trichoderma viride is one such candidate (Lin et al., 1994), which has reportedly reduced black scurf to 74.5% in a previous study (Schmiedeknecht, 1993). However, antagonistic activities of Trichoderma species are greatly affected by various soil factors including soil moisture (Griffin, 1972). There are conflicting reports about the role of moisture in affecting the activities of T. viride. Low soil water pressure promotes activities of the antagonist and brings about disease suppression (Knudsen and Bin, 1990). Conversely, Liu and Baker (1980) demonstrated that the survival of antagonistic Trichoderma species was greater in moist than in dry soils.
Interestingly, soil moisture also affects growth and sporulation of R. solani (Kouyeas, 1964) and is second to temperature in influencing the disease (Nagaich et al., 1974).
Manipulation of soil moisture to enhance the efficiency of biocontrol agent and therefore suppress the pathogen provides a potential practical disease management strategy. In view of this, the present studies were designed to test the interactive effect of soil moisture and T. viride on disease severity and to investigate the effect of soil moisture and T. viride on yield.
Materials and Methods
Isolation of R. solani Kühn
Infected potato tubers with symptoms of black scurf were collected from the local market. The pathogen, R. solani was isolated from these potatoes tubers as follows:
Tuber pieces (1 cm2), bearing sclerotia, were excised with a scalpel and surface sterilized with 0.1% solution of mercuric chloride (HgCl2) for 30 seconds, rinsed twice in sterile distilled water and blotted dry on sterilized filter papers (Whatman # 1). The disinfected pieces were then plated on potato dextrose agar (PDA) medium under aseptic conditions and later incubated at 25oC until colonies developed. Colonies thus formed were subsequently sub cultured for further studies. Identification was made according to the key reported by Barnett and Hunter (1972).
Revival of T. viride Pers
Colonies of T. viride (maintained on silica gel) were revived by plating on sterilized PDA medium, and later incubating at 25oC for one week. Colonies thus obtained were used for further studies as biocontrol agent.
Mass production of T. viride Pers
The biocontrol agent was grown on wheat bran for mass production to be used in vivo studies. Glass jars (4cm diameter) containing wheat bran immersed in distilled water (1:2v/v), were autoclaved at 121oC for 1 h. Plugs (1 cm2) of the biocontrol agent were plated on autoclaved wheat bran and incubated at 25oC for seven days. Bran subsequently covered with the biocontrol agent was used in greenhouse studies.
Greenhouse study
Treatments were consisted of five different moisture levels: 3%, 5%, 8%, 11% and 15% with R. solani alone or in combination with T. viride. The experiment was designed as split plot, with soil moisture in main plots and R. solani either alone or in combination with T. viride in subplots. Treatments were replicated five times.
Soil mixture (clay, sand, FYM 1:1:1w/w) was pasteurized at 75oC for 2 h, air dried on greenhouse benches for two weeks and then sieved to remove debris and stones.
Approximately 2kg soil was placed per pot (20cm dia). Soil was inoculated with R. solani @ one plate/pot alone or in combination with T. viride. The biocontrol agent was added @ 10 g/pot. Inoculation was performed 24 h before sowing, and soil mixed thoroughly in order to obtain a uniform bulk density as well as mixing of inoculum and the biocontrol agent.
Seed pieces of cv. Desiree, washed twice to remove dust and other contaminations, were left in a plastic tray for green sprouting in the greenhouse for two weeks prior to sowing. The sprouted tubers were planted in pots at a depth of 6cm. Based on different treatments; soil moisture was adjusted in each pot according to the equation (Ajmal et al., 2001):
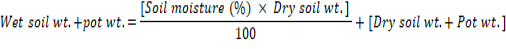
The pots were weighed daily till harvest and water added to maintain the desired moisture level. Urea @ 3g / pot was added following one month of sowing. Plants were maintained on greenhouse benches under natural daylight and temperature.
At harvest plant height from soil surface to the tip of the apical bud was recorded. Plants were cut near the soil surface and discarded. The underground portion of each plant was removed carefully from the soil, washed in running tap water, blotted dry and weighed.
Tubers dug out from each pot were counted and later weighed using an electric balance. Disease severity was based on a 0 to 5 scale (Ajmal et al., 2001) where 0= no symptoms on tubers; 1=less than 1% area affected; 2= 1-10% affected; 3=11-20% area affected; 4=21-50% area affected and 5 = 51% or more area affected.
Disease incidence was calculated using the equation.
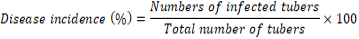
Data were statistically analyzed using statistix 8.1 software. For comparing means, LSD was applied wherever significant differences were observed among means.
Results and Discussion
Soil moisture and biocontrol agent had a significant interactive effect on disease severity, incidence, and tubers/plant. Similarly, a highly significant main effect of moisture levels was observed on tuber weight. On the other hand, there was no significant effect of either moisture levels or biocontrol agent on plant height and fresh root weight.
Disease severity
A significant (P=0.004) interactive effect of the fungal treatments and moisture levels on disease severity was observed. Application of biocontrol agent in pots maintained at 8% moisture level resulted in the least disease severity (1.27) (Table 1). A similar trend was observed in pots maintained at 11% moisture and inoculated with a combination of the pathogen and biocontrol agent (2.22). There was a reduction of 63.29% and 32.31% over control by T. viride at 8% and 11% moisture levels, respectively.
Disease incidence
A highly significant (P=0.0007) interactive effect of the fungal treatment and moisture levels on disease incidence was observed. Application of biocontrol agent in pots maintained at 8% moisture level resulted in the least disease incidence (53.73%) (Table 1). A similar trend was observed in pots maintained at 15% moisture and inoculated with a combination of pathogen and biocontrol agent (73.33%). There was a reduction of 43.33% and 9.84 % over control by T. viride at 8% and 15% moisture levels, respectively. Surprisingly, the lowest disease incidence (52.73%) was observed in pots maintained at 3% moisture level and unamended with the biocontrol agent.
Number of tubers per plant
A significant (P=0.04) interactive effect of the fungal treatments and moisture levels was observed on number of tubers/plant (Table 2). Application of biocontrol agent in pots maintained at 11% moisture level resulted in maximum number of tubers per plant (6.8). There was an increase of 52.94 % tubers/plant over control by T. viride at 11% moisture level.
Tuber weight
Moisture levels had highly significant (P=0.01) effect on tuber weight. The maximum tuber weight (54.31g) was obtained at 15% and minimum (12.48g) at 3% moisture level. Potatoes grown at 15% moisture had 77%
Table 1: Effect of soil moisture alone or in combination with Trichoderma viride on disease severity and % disease incidence of black scurf of potato.
|
Soil moisture level (% by wt.) |
Fungal treatment |
Disease severity |
% Decrease |
Disease incidence (%) |
% Decrease |
|
3 |
R. solani |
1.35 DE |
40.78 |
52.73 D |
44.49 |
|
R. solani + T. viride |
2.28 C |
- |
95 AB |
- |
|
|
5 |
R. solani |
2.10 CD |
15.66 |
90 AB |
3.57 |
|
R. solani + T. viride |
2.49 BC |
- |
93.33 AB |
- |
|
|
8 |
R. solani |
3.46 A |
- |
95AB |
- |
|
R. solani + T. viride |
1.27 E |
63.29 |
53.73 D |
43.33 |
|
|
11 |
R. solani |
3.28 AB |
- |
98.75 A |
- |
|
R. solani + T. viride |
2.22 C |
32.31 |
80.40BC |
18.58 |
|
|
15 |
R. solani |
2.25 C |
8.16 |
81.33 BC |
- |
|
R. solani + T. viride |
2.45 C |
- |
73.33 C |
9.84 |
LSD (P=0.004) for disease severity = 0.79; LSD (P=0.0007) for disease incidence = 16.50
LSD (P=0.04) for number of tubers per plant= 1.694; Means followed by different letter (s) in the same column are significantly different from one another according to Least Significant Difference (LSD) test.
Table 2: Effect of soil moisture alone or in combination with Trichoderma viride on number of tubers/plant in potato.
|
Soil moisture level (% by wt.) |
Fungal treatment |
Number of tubers /plant |
% Increase |
|
3 |
R. solani |
4.22 BC |
28.91 |
|
R. solani + T. viride |
3 C |
- |
|
|
5 |
R. solani |
2.6 C |
- |
|
R. solani + T. viride |
2.6 C |
0 |
|
|
8 |
R. solani |
4B C |
5 |
|
R. solani + T. viride |
3.8 BC |
- |
|
|
11 |
R. solani |
3.2 C |
- |
|
R. solani + T. viride |
6.8 A |
52.94 |
|
|
15 |
R. solani |
6.4 A |
18.75 |
|
R. solani + T. viride |
5.2 AB |
- |
more tuber weight than those at 3% moisture level (Figure 1). None of the fungal treatments had an effect on tuber weight. In addition, interaction between soil moisture and fungal treatments were also non-significant.
Fresh root weight and plant height
Both fresh root weight and plant height were not significantly affected by fungal treatments and moisture levels under the conditions of experiment. Likewise, interactive effects were not significant. Generally, the effect of soil moisture levels was inconsistent over the course of study.
Biological control of R. solani has much potential for disease management; though there are several problems with its practical implementation (Cook and Baker, 1983). Similarly, soil moisture condition in field greatly affects biocontrol of plant diseases (Griffin, 1972). Soil moisture may increase microbial activity and may have been associated with suppression of pathogen activity through competition (Gill et al., 2001). It may also significantly affect the health of potato crop.
The present studies revealed that sclerotial population or disease severity responded differently at different moisture levels in the presence or absence of biocontrol agent. Likewise T. viride was effective over a broad range of soil moistures. Lowest disease severity was observed at 8% moisture level in the presence of biocontrol agent. There was a highest reduction in disease severity over control was performed by T. viride at this moisture level. It may be attributed to the greatest efficiency of Trichoderma species in moist soil (Liu and Baker, 1980). However, at higher moisture level (15%), a lower reduction in disease severity over control was observed. It may be due to the reason that moist soil remained cool for longer period of time, which could have resulted in increased severity of black scurf (Powelson et al., 1993). Similarly the least disease incidence was also recorded at 8% moisture level with biocontrol agent and at 3% moisture level without biocontrol agent indicating that T. viride probably could not thrive under such low moisture conditions (Liu and Baker, 1980). Similar findings have also been reported by Ajmal et al. (2001) who observed lowest incidence and severity of the disease at 8.45% soil water pressure followed by 5.63% soil water pressure in their study on the effect of soil water pressure on the severity of black scurf in potato. In a study on the effect of soil moisture and fumigant in controlling damping off of peas Donald et al. (1970) also obtained the most effective control in moderately moist soil (10% water) than wet (37%water) or very dry soil (2%water).
Reduction in yield at low soil moisture level may be attributed to depletion of soil moisture than normal quantity without its replenishment. Growth slows down and may even cease with a 10% decrease in water content (David, 1993). On the other hand, higher yield at high moisture level was due to the availability of sufficient moisture for the growth of plants. This also allowed the plants to withstand disease at all growth stages.
The biocontrol agent favored maximum number of tubers per pot. This may have been due to lower disease severity and incidence at these moisture levels. Similarly, sufficient moisture was also available for proper growth.
The highest fresh root weight was achieved at comparatively higher moisture level up to a point but decreased with further increase in moisture. The results are in agreement with those of Dorrance et al. (2003) who demonstrated that root weight in soybean was greater with more stands at 50% and 75% than at 25 or 100% water holding capacity.
Conclusions and Recommendations
In light of the findings of the present studies, it can be inferred that the interactive effect of soil moisture and Trichoderma viride on control of black scurf was most effective at 8% moisture level. There was no interactive effect of biocontrol and moisture on yield. The highest yield was obtained at 15% moisture level.
Novelty Statement
Different soil moisture levels were tested to suppress the virulence of Rhizoctonia solani and enhance the performance of Trichoderma viride as biocontrol agent.
Author’s Contribution
Azra Nadeem: Designed and conducted the experiment and wrote the manuscript.
Shaukat Hussain: Provided conceptual frame work and helped in development of the manuscript.
Saeedullah: Helped in culture purification and mass production of inoculum.
Asma Akbar: Helped in writing of the manu-script.
Zahoor Ahmad: Helped in lab work.
Maryam Tariq: Recorded lab and green house data.
Robina Karim: Analyzed data.
Muhammad Huzaifa: Provided susceptible variety and recorded greenhouse dated.
Conflict of interest
The authors have declared no conflict of interest.
References
Ajmal M., S. Ahmad and S. Hussain. 2001. Effect of soil moisture on black scurf disease and yield of potato. Pak. J. Biol. Sci., 4: 150-151.
Barnett H.L. and B.B. Hunter. 1972. Illustrated Genera of Imperfect Fungi. 3rd ed Burgess Publishing Co. Minneopolis, Minnesota, USA. 203pp.
Carling D.E. and R.H. Leiner. 1990. Effect of temperature on virulence of Rhizoctonia solani and other Rhizoctonia on potato. Phytopathology, 80: 930–934. https://doi.org/10.1094/Phyto-80-930
Cook R.J. and K.F. Baker. 1983. The Nature and Practice of Biological Control of Plant Pathogens.
David C. 1993. Water Management. Pages 67-76 In: Potato Health Management. (Randell C. Rowe ed). Plant Health Management Series, APSP St. Paul Minnesota.
Donald E.M., B.J. Moore and F. Abu-El-Haj. 1970. Soil moisture effect on control of Pythium ultimum or Rhizoctonia.solani with methyl bromide. Phytopathology, 61: 194-196. https://doi.org/10.1094/Phyto-61-194
Dorrance A.E., M.D. Kleinhenz., S.A. McClure and N.T. Tuttle. 2003. Temperature, moisture and seed treatment effects on Rhizoctonia solani root Rot of soybean. Plant Dis., 87: 533-538. https://doi.org/10.1094/PDIS.2003.87.5.533
Gill J.G., J.S. Sivasithamparam and K.R.J. Smettem. 2001. Soil moisture affects disease severity and colonization of wheat roots by R. solani AG-8. Department of Soil Sciences and Plant Nutrition, Uni. Of Western Australia. https://doi.org/10.1016/S0038-0717(01)00041-4
Gould W.A. 1999. Potato Production, Processing and Technology. CTI Publications, Inc. Maryland. https://doi.org/10.1533/9781845696122
Griffin D.M. 1972. Ecology of Soil Fungi. Chapman and Hall, London. 71–85.
Jager G.H., J.G. Velvis, A. Lamers, A. Mulder and J. Roosjen. 1991. Control of Rhizoctonia solani by biological, chemical, and integrated control measures. Potato Res., 34: 269–284. https://doi.org/10.1007/BF02360500
Knudsen G.R. and L. Bin. 1990. Effects of temperature, soil moisture and wheat bran on growth of Trichoderma harzianum from alginate pellets. Phytopathology, 80: 724-727. https://doi.org/10.1094/Phyto-80-724
Kouyeas V. 1964. An approach to the study of moisture relation of soil fungi. Plant Soil., 20: 351-363. https://doi.org/10.1007/BF01373825
Kumar, M., A. Kumari, J. K. Singh, S. Kumar and R. Niwas. 2018. Influence of soil temperature, moisture and planting depth on black scurf development in potato (Solanum tuberosum L.). J. Agrometeorol., 20 (4): 342-344.
Lin A., T.M. Lee and J.C. Rern. 2014. Tricholin, a new antifungal agent from Trichoderma viride, and its action in biological control of Rhizoctonia solani. J. Antibio., 47(7): 799-805.
Liu S. and R. Baker. 1980. Mechanism of biological control in soil suppressive to Rhizoctonia solani. Phytopathology, 70: 404-412. https://doi.org/10.1094/Phyto-70-404
Malik, N.J. 1995. Potatoes in Pakistan: A handbook. Pak-Swiss Potato Development Project, Pakistan Agricultural Research Council.
Nagaich B.B., N.L. Shekhawat, S.M. Khurana and S.K. Bhattachanyra. 1974. Pathological problems of potato cultivation in India. J. India. Potato Assoc., 5: 32-44.
Powelson M.L., K.B. Johnson and R.C. Randall. 1993. Management of disease caused by soilborne pathogens. Pages 149-158 In: Potato Health Management (Randal C. Rowe ed). Plant Health Management Series, APSP St. Paul Minnesota.
Rauf, C.A., M. Ashraf and I.Ahamd. 2007. Management of black scurf disease of potato. Pak. J. Bot., 39(4): 1353-1357.
Ristaino J.E. and G.C. Papavizas. 1985. Biological control of Rhizoctonia stem canker and black scurf of potato. Phytopathology, 75: 560–564. https://doi.org/10.1094/Phyto-75-560
Schmiedeknecht G. 1993. Biological control of Rhizoctonia solani Kuhn on potatoes by microbial antagonists. Arch. Phytopathol. Plant Prot., 28: 311-320.
Secor G.A. and N.C. Gudmestad. 1999. Managing fungal diseases of potato. Am. J. Plant Pathol., 21: 213–221. https://doi.org/10.1080/07060669909501184
Tsror, L., R. Barak and B. Sneh. 2000. Biological control of black scurf on potato under organic management. Crop Prot., 20:145-150. https://doi.org/10.1016/S0261-2194(00)00124-1
To share on other social networks, click on any share button. What are these?







