Phytochemical Analysis and Biological Evaluation of Methanolic Leaf Extract of Corylus jacquemontii Decne
Research Article
Phytochemical Analysis and Biological Evaluation of Methanolic Leaf Extract of Corylus jacquemontii Decne
Altaf Ur Rahman1, Muhammad Ajmal Khan2*, Ali Hazrat3, Awais Farid4, Muhammad Yahya3* and Inam Ullah5
1Department of Botany Hazara University Mansehra, Khyber Pakhtunkhwa, Pakistan; 2Center of Biotechnology and Microbiology, University of Peshawar, Peshawar, Khyber Pakhtunkhwa, Pakistan; 3Department of Botany, University of Malakand, Chakdara Dir Lower, Khyber Pakhtunkhwa, Pakistan; 4Division of Environment and Sustainability, Hong Kong University of Science and Technology, Hong Kong; 5Department of Zoology, Abdul Wali Khan University, Mardan, Khyber Pakhtunkhwa, Pakistan.
Abstract | There is a great concern for healthcare community in antibiotic resistance and evolution of novel bacterial strains. Numerous plants act as a source of novel compounds that could be used to develop new drugs. Therefore, the current study was aimed to investigate the methanolic extract of Corylus jacquemontii for phytochemical, antibacterial, antioxidant potential. The methanolic fraction of C. jacquemontii was evaluated for quantitative phytochemical constituents. The antibacterial efficacy was investigated via well diffusion method by using a concentration of 100 µg/ml. Furthermore, the antioxidant potential of extract was estimated at various concentration (50, 100, 150, 200, 250 µg/ml) through 2, 2-Diphenyl -1- picryl-hydrazyl (DPPH) assay (150 µg/ml). Results showed that the methanolic extract of C. jacquemontii is a rich source of many phytochemically active compounds such as alkaloids content (3.60%), flavonoids (3.40%) and terpenoids 2.30%. The methanolic extract of C. jacquemontii depicted promising antibacterial potential towards various tested bacterial strain in order of P. aeruginosa > E. coli > S. typhi > MRSA comparable to vancomycin. The highest percent mean growth inhibition (MGI) was observed against P. aeruginosa of 90.5±4.3 compared to vancomycin (61.26±6.31). The Zone of Inhibition (ZOI) noted against P. aeruginosa was 20 mm compared to vancomycin (17 mm). The quantitative test antioxidant activity of the methanolic fraction of C. jacquemontii at 250 µg/ml revealed the best antioxidant potential when the DPPH concentration of 150 µg/ml was used. The result showed that extract had a significant effect on antioxidant activity. Therefore, the present study supports the effectiveness of methanolic leaf extract of C. jacquemontii in traditional medicine and may be investigated further for mode of action on molecular basis.
Received | August 26, 2021; Accepted | March 14, 2023; Published | June 21, 2023
*Correspondence | Muhammad Ajmal Khan and Muhammad Yahya, Center of Biotechnology and Microbiology, University of Peshawar, Peshawar, Khyber Pakhtunkhwa, Pakistan; Department of Botany, University of Malakand, Chakdara Dir Lower, Khyber Pakhtunkhwa, Pakistan; Email: ajmal.mb137@gmail.com, m.yahyabotanist@gmail.com
Citation | Rahman, A., M.A. Khan, A. Hazrat, A. Farid, M. Yahya and I. Ullah. 2023. Phytochemical analysis and biological evaluation of methanolic leaf extract of Corylus jacquemontii decne. Sarhad Journal of Agriculture, 39(2): 564-572.
DOI | https://dx.doi.org/10.17582/journal.sja/2023/39.2.564.572
Keywords | Antibacterial activity, Antioxidant activity, Alkaloids, C. jacquemontii, Flavonoids, Terpenoids
Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
In last 30 years antimicrobial agents had played an extremely vital role in management of many deadly infectious diseases (Nasrullah et al., 2012), but the emergence of microbial resistance and evolution of different strains have reduced their efficacy (Zaman et al., 2017). Infections caused by these resistant strains have serious consequences such as increased hospitalization, higher cost and escalated mortality and morbidity rates. According to Centre for Disease Control (CDC), resistant bacteria infect 2 million people in United States per year and it was estimated that direct health care cost was $20 billion and loss in productivity was $35 billion (CDC, 2013). The consequences were more drastic in low-income countries where people lack efficient surveillance and diagnostics. They cannot afford antimicrobials because of their limited income. It was estimated that if no fruitful efforts were made to intervene new drugs, death toll will reach to 10 million with cost of $100 trillion till 2050 in the world (Solomon and Oliver, 2014; Rather et al., 2017; Shehadeh et al., 2016; Courvalin, 2016; Morehead and Scarbrough, 2018). Therefore, the quest for effective and affordable antimicrobial agents were increasing day by day in health care especially in developing countries where deaths were mainly due to infectious diseases and resistance to many antibiotics (Awouafack et al., 2013; Srivastava et al., 2014; Theuretzbacher and Mouton, 2011; Walsh and Toleman, 2012). The natural resources such as plants are ultimately an important part to be screen for effective and novel drug development to tackle the problem of high cost and drug resistance.
The use of natural products and secondary metabolites originated from living system, mainly from plants had shown a boost to health care since ancient time. Furthermore, the modern medical science success rate is also dependent on the drugs that are acquired from natural resources. The limitless application of natural resources in treating many human diseases upsurge its usage due to its low cost and no side effects. WHO reported that nearly 65-80 percent population of the world rely on herbal medicine for treating different diseases (Gurinder and Daljit, 2009). Many studies have reported that plants contain bioactive compounds such as alkaloids, flavonoids, terpenoids and glycosides, that have a strong propensity for microbial agents (Obafemi et al., 2006). Plant extracts have previously been used on a variety of bacterial species, including Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli and were found at various levels to be effective (Mostafa et al., 2018). Antibacterial activity of Persea americana against Bacillus cereus was observed at concentration of 10 and 25 mg/ml by using butanolic stem bark extracts (Akinpelu et al., 2015). Antimicrobial activity against twelve pathogenic microorganisms in four separate plant extracts were studied and found that most extracts could minimize the growth of pathogenic microorganisms (Manandhar et al., 2019).
C. jacquemontii (Decne), belongs to corylaceae family, and is commonly called Thangi or Thankoli. A deciduous tree with a height of 21m. The flowering stage begins from April to May, and from September to October. It is one of the important and economical species in the western Himalayan region. The species of this plant is found in various countries, including Pakistan, High above sea level in Afghanistan and in western Nepal at 1900-3000 m. This plant is also used to guard against inflammation, antioxidant, anticancer and cardiovascular. The seeds are also used as a fruit and the local collectors sell it on the market for economic purposes as it ripens (Bhalodia and Shukla, 2011). The Current study aims to evaluate phytochemical, antibacterial, antioxidant potential.
Materials and Methods
Study area
The current work was conducted in the Department of Botany, Hazara university Mansehra, Pakistan. The Department provide all the facilities for the current research work.
Plant material collection and authentication
C. jacquemontii was collected from District Kohistan Khyber Pakhtunkhwa, Pakistan. The plant was identified with the assistance of flora from Pakistan, taxonomists, and various pictorial guides. Voucher specimen No- 56094 were allotted to the collected plant and deposited in the Herbarium of Department of Botany Hazara University, Mansehra, KPK, Pakistan. Leaves of C. jacquemontii was washed meticulously with clean tap water for soil waste removal followed by surface sterilization with 1% perchloric acid and 70% ethanol and dry in the shade afterward. Dry plant material was crushed through electrical grinder into powder form and stored in bottles for future experiments (Iftikhar et al., 2020).
Extraction
Hot continuous extraction or Soxhlet extraction method was used for the phytochemicals extraction from C. jacquemontii leaves. This technique involved by putting the crushed plant material in the Soxhlet apparatus’ thimble chamber. The extraction solvent, Methanol was heated in the flask and vaporized into the sample thimble. It is then condensed in the condenser and dripped back. All the liquid content when reached to the siphon arm were emptied in flask and the process was repeated (Konga et al., 2017). Briefly, methanol (600ml) was used to pulverized and soaked 200g of plant material for 48 hours in Soxhlet apparatus. The extract was dried after being concentrated by evaporation at 70 °C for 8 hours via rotary evaporator (Sengul et al., 2009). Further fractionation was performed into n-hexane and chloroform and evaporated at 40°C with Rotary Evaporator (Rahman et al., 1997; Nisar et al., 2011).
Quantitative phytochemical screening
Quantitative test for alkaloids: Quantitative test for alkaloids was performed followed by the previously reported procedure with slight modification (Harborne, 1992). Briefly, 5g of leaves powdered was taken and 200ml of methanol was added to it and kept for 4 hours. Concentrated ammonium hydroxide was added to the mixture and filtered. Finally, the precipitate was weighted according to the given formula.
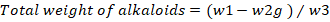
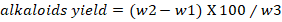
w2= Petri plate with alkaloids weight; w3= Quantification of initial plant sample weight.
Quantitative test for flavonoids: Bohm and Kocipai-Abyazan (1994) protocol was used for the quantitative determination of flavonoids content in the methanolic extract of C. jacquemontii. Briefly, 10g leaves powder was taken in a beaker and 80% methanol (80mL) was added and the mixture was filtered. The process was repeated again and again until complete purity. Finally, the extract was transferred to petri dish for drying. The dry extract was then weighted according to the formula.
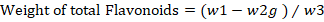
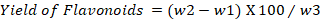
w2= Flavonoid’s weight with crucible; w3= Estimation of initial weight of plant sample.
Quantitative test for terpenoids: The quantitative analysis for terpenoids was performed according to Indumathi et al. (2014) with some modifications. Briefly, 10g of leaves powder was soaked in 9ml of methanol. The extract was then transferred to another vial for drying. The % of terpenoids were then calculated by the following formula.
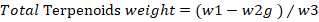
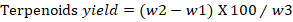
w2= weight of crucible with Terpenoids; w3= initial weight of plant sample taking for estimations.
Determination of antibacterial activity
Test organisms: The different pathogenic bacterial strains used in the current study containing three Gram negative strains Escherichia coli (E. coli) (ATCC 25922), Salmonella typhi (ATCC 39183), Pseudomonas aeruginosa (ATCC 27853), and 1 Gram-positive strain S. aureus (ATCC 29213). These bacterial strains were cultured from original stock kept at -70°C. All the bacterial strains were provided by the center of biotechnology and microbiology, University of Peshawar and all the experiments were performed in accordance with Clinical and Laboratory Standards Institute (CLSI) guidelines (Wayne, 2014). Antibacterial activities of the extract were performed through broth dilution method (Stalons and Thornsberry, 1975) and well diffusion method (Wiegand et al., 2008).
Broth dilution method
Stalons and Thornsberry (1975), method was used to evaluate the antibacterial potential of methanolic extract of C. jacquemontii against the test microorganisms. Bacterial culture was prepared in Luria broth (LB) media and incubated for 12 h at 37 °C. After overnight of incubation the culture was diluted to 104 colony forming units (CFU) with fresh LB medium and methanolic extract (100 µg/ml) was added and plates were further incubated for 12 hr at 37°C. The treated and untreated cultures were then compared for growth. Vancomycin (30µg disc) was used as a standard control. The percentage mean growth inhibition (MGI) was calculated by using the formula from three independent experiments.
% MGI = [(dc − dt)/dc] x 100
Well diffusion method
The prepared media (1000 ml distil water containing 28 g Nutrient agar) were sterilely poured onto the Petri plates. The homogenization of the bacterial culture was done in 8 ml broth medium (13 g Nutrient Broth/1000 ml distill water) in shaking water bath (37˚C) for 16 hours at a speed of 200 rpm. After overnight of incubation the bacteria cultured were compared for turbidity (0.5 McFarland turbidity standards) and diluted accordingly. Bacteria culture was then spread on plate via glass spreader and kept at 37˚C for setting of culture. With the help of cork borer wells were formed and 6μl of plant extract having concentration of 100 µg/ml was added to the wells. As a negative control, 10% dimethyl sulfoxide (DMSO) was used (Gonelimali et al., 2018). Vancomycin (30 µg disc) was used as a standard. The minimal zone of inhibition (zoi) around each disc was measured in millimeters (mm) following 24 hours of incubation.
Antioxidant activity
A quantitative approach with certain modifications was used to test the extract’s antioxidant properties (Sengul et al., 2009). 2, 2’-Diphenyl-1-picrylhydrazyl (DPPH) was used, and 6.6 ml of it was dissolved in 15 ml tubes while they were wrapped in aluminum foil. The methanolic leaf extract of C. jacquemontii was obtained in separate tubes and combined with 1 mM of DPPH at various doses (50, 100, 150, 200, and 250 µg/ml). Each tube’s color was checked, and the antioxidant activity of the extract was calculated based on the color changed.
Statistical analysis
The experimental data of antibacterial activity was analyzed by mean±SD from triplicate experiment by using GraphPad prism 8.1 software. * p < 0.05 was considered statistically significant calculated by student t-test (Student, 1908).
Results and Discussion
Quantitative phytochemical analysis
The presence of different phytochemical compounds/secondary metabolites aided in plant antimicrobial activity due to its biologically active nature. The methanolic extract of C. jacquemontii revealed the presence of alkaloids (3.60%), flavonoids (3.40%) and terpenoids (2.30%) (Figure 1).
Antibacterial potential of C. jacquemontii
Broth dilution method: The antibacterial potential of methanolic extract (100 µg/ml) of C. jacquemontii was performed on the test microorganism by accessing the % MGI with comparison to standard antibiotic vancomycin. The results showed that the leaves methanolic extract exhibited invitro bactericidal property. The growth of tested microorganism was hampered and the % inhibition was shown in Table 1 and Figure 2. The results showed % growth inhibition in order of P. aeruginosa > E. coli > S. typhi > MRSA.
Well diffusion method
Plant extracts have bioactive compounds or secondary metabolites which are linked to antibacterial activities. The methanolic extract of C. jacquemontii were further
Table 1: % mean growth inhibition (% MGI) of various bacterial strains in the presence of methanolic leaves extract of C. jacquemontii at (100 µg/ml).
|
Bacterial strains |
Extract % MGI |
Vancomycin (30 µg disc) %MGI |
|
Pseudomonas aeruginosa (P.A) (ATCC 27853) |
90.5±4.3 |
61.26±6.31 |
|
Escherichia coli (E. coli) (ATCC 25922) |
76.86±4.4 |
74.5±1.7 |
|
Salmonella typhi (S.T) (ATCC 39183) |
60.85±2.7 |
56.03±2.9 |
|
Methicillin resistant Staphylococcus aurous (MRSA) (ATCC 29213) |
50.9± 2.6 |
57.4±4.17 |
tested against four pathogenic bacteria using disc diffusion assay. Based on ZOI production, C. jacquemontii methanolic extract at 100 µg/ml demonstrated very efficient antibacterial activity. The antibacterial activities depicted by the extract showed a ZOI from 15-20 mm against tested bacterial strain. The results were summarized in the Figure 3 and Table 2. The maximum inhibitory activity of the extract against bacteria is in the order of P. aeruginosa > E. coli > S. typhi > MRSA.
Table 2: C. jacquemontii (100 µg/ml) antibacterial activity displaying zone of inhibition (mm) and standard antibiotic Vancomycin (30 µg disc).
|
Bacterial strains |
Extract (ZOI) |
Antibiotic (ZOI) |
|
Pseudomonas aeruginosa(P.A) |
20±1.167 mm |
18 mm |
|
Escherichia coli (E. coli) |
18±2.306 mm |
16 mm |
|
17±2.306 mm |
14 mm |
|
|
15±2.306 mm |
13 mm |
Quantitative antioxidant activity of methanolic leaf extract of C. jacquemontii
The methanolic extract of C. jacquemontii were evaluated for quantitative antioxidant activity through DPPH scavenging assay. Extract’s scavenging activities were compared with the activity of standard control, ascorbic acid. The extract showed a dose dependent color change, showing the amount of extract reacting with DPPH. At 150 µg/ml concentration of DPPH and 250 µg/ml concentration of extract dark black color was observed. These results demonstrated that the amount of extract will significantly affect the antioxidant activity represented by color changed in a dose dependent manner (Table 3).
An important aromatic plant with nutritional and medicinal benefits is Corylus jacquemontii (Decne). This plant is highly abundant in the District Kohistan Khyber Pakhtunkhwa, Pakistan and requires very little maintenance. In the current study, the phytochemical content, antibacterial, and antioxidant properties of the methanolic leaf extract of C. jacquemontii were investigated. Using a previously described approach, secondary metabolites such as alkaloids (3.60%), flavonoids (3.40%), and terpenoids (2.30%) were found in the methanolic fraction of C. jacquemontii, which demonstrated the highest level of phytochemical activity. Gymnema sylvestre were quantitively evaluated and showed the similar secondary metabolites (Kumar and Patra, 2017). The presence of different biologically active compounds such as alkaloids, flavonoids and terpenoids in plant extracts aided into plant different antimicrobial activities, hence use in traditional medicine (Chukwuka et al., 2011; Khan et al., 2021). Based on preliminary studies alkaloids and flavonoids are known to be biological response modifiers due to their role in modification of viruses, allergens, carcinogens. Invitro studies have shown their potential as an antimicrobial, anticancer, anti-allergic, anti-inflammatory (Spencer, 2008; Thawabteh et al., 2019). Alkaloids are heterocyclic nitrogenous group of compounds having antimicrobial potential.
Table 3: Antioxidant activity by qualtitative method for C. jacquemontii methanolic leaves extract.
|
S. No |
Conc of plant extract (µg/ml) |
DPPH Conc (µg/ml ) |
Extract color |
Control |
Color of ascorbic acid |
|
1 |
50 |
150 |
Dark Red |
Ascorbic acid |
Slightly white |
|
2 |
100 |
150 |
Red |
Ascorbic acid |
Slightly white |
|
3 |
150 |
150 |
Slightly red |
Ascorbic acid |
Slightly white |
|
4 |
200 |
150 |
Slightly black |
Ascorbic acid |
Slightly white |
|
5 |
250 |
150 |
Dark black |
Ascorbic acid |
Slightly white |
The major mode of action of alkaloids is their intercalation with DNA (Setzer et al., 2006). Flavonoids are considered natural antioxidants and antimicrobial agents by their ability to interact with bacterial cellular membrane, protein, and cell wall.
Similarly, the methanolic fraction of C. jacquemontii have good antibacterial activity against P. aeruginosa, and have a 20 mm zone of inhibition, as compared to the standard antibiotic, which have 18 mm zone of inhibition. The antibacterial potential tested with Broth dilution method also showed highest activity against P. aeruginosa with %MGI of 90±4.3 compared to vancomycin (61.26±6.31). Our results are closely related with a previous study (Ceylan et al., 2013). They performed antibacterial activity of C. colurna leaf extract using methanol, dimethyl sulfoxide, distilled water, and petroleum ether. These extracts were also examined using the disc diffusion method to evaluate various Gram positive and Gram-negative strains. Another study also supported our results by evaluating the various extract of Triphala against different bacterial species and their antioxidant potential (Parveen et al., 2018).
Similarly, the quantitative antioxidant activity of methanolic fraction of C. jacquemontii showed a dose dependent color changed, higher the amount of plant extract higher is the color change and hence higher the antioxidant activity. When DPPH was used at a concentration of 150 µg/ml and the extract was used at 250 µg/ml, a greater color shift was seen. This concentration showed a dark black color. This showed that extract amount has significant on antioxidant activity. Our results are supported by another study which evaluated the antioxidant activity on C. colurna by using the similar protocol (Riethmueller et al., 2014). The presence of preferential quantity of different secondary metabolites in methanolic extract C. jacquemontii and its antibacterial potential as revealed in current study will further directed to emphasize on active compound purification.
Conclusions and Recommendations
C. jacquemontii is widely used as an anti-inflammatory, anticancer, antioxidant and cardio disorder protective remedy. Recent study showed that the methanolic leaves extract of the plant have promising phytochemical, antibacterial, and antioxidant activity. The main aim of current study is to focus on the medicinal potential of C. jacquemontii and the medicinal value of this plant suggest that future research should be conducted in a manner to keep in mind the properties of such wonder plant, while analyzing, isolating, characterizing the active compounds present in it.
Acknowledgment
The authors are thankful to the Department of Botany Hazara University Mansehra, Pakistan and Department of Botany, University of Malakand, Chakadra Dir Lower to provide all lab work facilities during research work.
Novelty Statement
This is the first study reported on the biological evaluation on Corylus jacquemontii Decne., collected from District Kohistan Khyber Pakhtunkhwa, Pakistan.
Author’s Contribution
Altaf ur Rehman, Muhammad Yahya, Muhammad Ajmal Khan: Conception and design.
Altaf Ur Rehman, Muhammad Yahya, Muhammad Ajmal Khan: Development of methodology.
Altaf Ur Rehman, Muhammad Ajmal Khan, Inam Ullah: Acquisition of the data.
Muhammad Ajmal Khan: Analysis and interpretation of data.
Altaf ur Rehman, Muhammad Ajmal Khan, Muhammad Yahya: Writing, review, and/or revision of the manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References
Akinpelu, D.A., O.A. Aiyegoro, O.F. Akinpelu and A.I. Okah. 2015. Stem bark extract and fraction of Persea americana (Mill) exhibits bactericidal activities against strains of Bacillus cereus associated with food poisoning. Molecules, 20: 416–429. https://doi.org/10.3390/molecules20010416
Awouafack, M.D., L.J. McGaw, S. Gottfried, S. Mbouangouere, R. Tane, P. Spiteller, M. and J.N. Eloff. 2013. Antimicrobial activity and cytotoxicity of the ethanol extract, fractions and eight compounds isolated from Eriosema robustum (Fabaceae). Com. Altern. Med., 13(1): 1-9. https://doi.org/10.1186/1472-6882-13-289
Bhalodia, N.R. and V.J. Shukla. 2011. Antibacterial and antifungal activities from leaf extract of Cassia fistula an Ethnomedicinal plant. J. Adv. Pharm. Technol. Res., 2(2): 104-109. https://doi.org/10.4103/2231-4040.82956
Bohm, B.A. and R. Kocipai-Abyazan. 1994. Flavonoids and condensed tannins from the leaves of Vaccinum raticulation and Vaccinum calyimium. Pac. Sci., 48: 458-463.
CDC (Centers for Disease Control and Prevention), 2013. Antibiotic resistance threats in the United States.Available at: https://www. cdc. gov/drugresistance/pdf/ar-threats-2013–508. pdf.” Accessed March 16 (2023).
Ceylan O., M.D. Sahin and S. Avaz. 2013. Antibacterial activity of Corylus colurna L. (Betulaceae) and Prunus divaricata Ledep. subsp. divaricata (Rosaceae) from Usak, Turkey. J. Agric. Sci., 19(6): 1204-1207.
Chukwuka, K.S, J.O, Ikheloa, I.O. Okonko, J.O. Moody and T.A. Mankinde. 2011. The antimicrobial activities of some medicinal plants on Escherichia coli as an agent of diarrhea in livestock. Adv. App. Sci. Res., 2(4): 37–48.
Courvalin, P., 2016. Why is antibiotic resistance a deadly emerging disease? Clin. Microbiol. Infect., 22(5): 405–407. https://doi.org/10.1016/j.cmi.2016.01.012
Gonelimali, F.D., J. Lin, W. Miao, J. Xuan, F. Charles, M. Chen and S.R. Hatab. 2018. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front. Microbiol., 9: 1639. https://doi.org/10.3389/fmicb.2018.01639
Gurinder, J.K. and S.A. Daljit. 2009. Antibacterial and phytochemical screening of Anethum graveolens, Foeniculum vulgare and Trachyspermum ammi. Complement. Altern. Med., 9: 30-10.1186/1472-6882-9-30. https://doi.org/10.1186/1472-6882-9-30
Harborne, J.B., 1992. A guide to modern technique of plant analysis. Chapman and Hill, London. Phytochem. Met., 279.
Hazrat, A., M.O. Nisar, J. Shah and S.H. Ahmad. 2011. Ethnobotanical study of some elite plants belonging to Dir, Kohistan valley, Khyber Pakhtunkhwa, Pakistan. Pak. J. Bot., 43(2): 787-795.
Iftikhar, K., M. Luqman and M.S. Mahmood. 2020. Phytochemical analysis of leaf extract of Calliandra haematocephala and in vitro antibacterial activity against food borne bacteria. J. Glob. Innov. Agric. Soc. Sci., 8: 26-29. https://doi.org/10.22194/JGIASS/8.883
Indumathi, C., G. Durgadevi, S. Nithyavani, and P.K. Gayathri. 2014. Estimation of terpenoid content and its antimicrobial property in Enicostemma litorrale. Int. J. Chem. Tech. Res., 6(9): 4264–4267.
Khan, B., A. Ullah, M.A. Khan, A. Amin, M. Iqbal. 2021. Anti-hyperglycemic and anti- hyperlipidemic effects of a methanolic extract of Debregeasia salicifolia in Alloxan-induced diabetic albino mice. Braz. J. Biol., 84. https://doi.org/10.1590/1519-6984.251046
Konga, A.K., A.S. Muchandi and G.P. Ponnaiah. 2017. Soxhlet extraction of Spirogyra sp. algae: An alternative fuel. Biofuels, 8(1): 29-35. https://doi.org/10.1080/17597269.2016.1196328
Kumar, A. and S. Patra. 2017. Qualitative and quantitative analysis of secondary phytochemical in Gymnema sylvestr. Indian J. Sci. Res., 12(2): 150-156.
Manandhar, S., S. Luitel and R.K. Dahal. 2019. In vitro antimicrobial activity of some medicinal plants against human pathogenic bacteria. J. Trop. Med., 2019(5): 1-5. https://doi.org/10.1155/2019/1895340
Morehead, M.S. and C. Scarbrough. 2018. Emergence of global antibiotic resistance. Prim. Care Clin. Off. Pract., 45(3): 467–484. https://doi.org/10.1016/j.pop.2018.05.006
Mostafa, A.A., A.A. Al-Askar, K.S. Almaary, T.M. Dawoud, E.N. Sholkamy and M.M. Bakri. 2018. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J. Biol. Sci., 25: 361-366. https://doi.org/10.1016/j.sjbs.2017.02.004
Nasrullah., K. Suliman, M. Rahman, M. Ikram, M. Nisar and I. Khan. 2012. Screening of antibacterial activity of medicinal plants. Int. J. Pharm. Sci. Rev. Res., 14: 25-29.
Newsroom, C.D.C., 2014. Untreatable: Report by CDC details today’s drug-resistant health threats.
Nisar, M., W.A. Kaleem, M. Qayum, I.K. Marwat, M. Zia-UlHaq and I. Ali. 2011. Biological screening of Zizyphus oxyphylla Edgew stem. Pak. J. Bot., 43(1): 311-317.
Obafemi, C.A., D.A. Akinpelu, O.O. Taiwo and A. Adeloye. 2006. Antimicrobial activity of solvent extracts of Terminalia catappa Linn leaves. Ife J. Sci., 8(1): 29-33. https://doi.org/10.4314/ijs.v8i1.32198
Parveen, R., T.N. Shamsi, G. Singh, T. Athar and S. Fatima. 2018. Phytochemical analysis and in-vitro biochemical characterization of aqueous and methanolic extract of Triphala, a conventional herbal remedy. Biotech. Rep., 17: 126-136. https://doi.org/10.1016/j.btre.2018.02.003
Rahman, A., R.M. Khan, I.M. Choudhary and Z.M. Iqbal. 1997. Steroidal Alkaloids from Sarcococca saligna. Phytochemistry, 45: 861-864. https://doi.org/10.1016/S0031-9422(97)00069-1
Rather, I.A., B.C. Kim, V.K. Bajpai and Y.H. Park. 2017. Self-medication and antibiotic resistance: Crisis, current challenges, and prevention. Saudi J. Biol. Sci., 24(4): 808–812. https://doi.org/10.1016/j.sjbs.2017.01.004
Riethmueller, E., G. Toth, A. Alberti, M. Sonati and A. Kery. 2014. Antioxidant activity and phenolic composition of Corylus colurna. Nat. Prod. Commun., 9(5): 679-682.
Sengul, M., H. Yildiz, N. Gungor, B. Cetin, Z. Eser and Ercisli. 2009. Total phenolic content, antioxidant, and antimicrobial activities of some medicinal plants. Pak. J. Pharm. Sci., 22(1).
Setzer, W.N., B. Vogler, L.J. Cseke, A. Kirakosyan, P.B. Kaufman, S.L. Warber, J.A. Duke and H.L. Brielmann. 2006. Bioassays for activity. Nat. Prod. Plants, pp. 390-413.
Shehadeh, M.B., G. Suaifan and E.A. Hammad. 2016. Active educational intervention as a tool to improve safe and appropriate use of antibiotics. Saudi Pharm. J., 24(5): 611–615. https://doi.org/10.1016/j.jsps.2015.03.025
Solomon, S.L. and K.B. Oliver. 2014. Antibiotic resistance threats in the United States: Stepping back from the brink. Am. Fam. Physician., 89(12): 938.
Spencer, J.P.E., 2008. Flavonoids: Modulators of brain function? Br. J. Nutr., 99(E-S1): ES60–ES77. https://doi.org/10.1017/S0007114508965776
Srivastava, J., H. Chandra, A.R. Nautiyal, and S.J. Kalra. 2014. Antimicrobial resistance (AMR) and plant-derived antimicrobials (PDA ms) as an alternative drug line to control infections. Biotech., 4(5): 451-460. https://doi.org/10.1007/s13205-013-0180-y
Stalons, D.R. and C. Thornsberry. 1975. Broth-dilution method for determining the antibiotic susceptibility of anaerobic bacteria. Antimicrob. Agents Chemother., 7(1): 15-21. https://doi.org/10.1128/AAC.7.1.15
Student, 1908. The probable error of a mean. Biometrika, pp. 1-25. https://doi.org/10.2307/2331554
Thawabteh, A., S. Juma, M. Bader, D. Karaman, L. Scrano, S.A. Bufo and R. Karaman. 2019. The biological activity of natural alkaloids against herbivores, cancerous cells and pathogens. Toxins, 11(11): 656. https://doi.org/10.3390/toxins11110656
Theuretzbacher, U. and J.W. Mouton. 2011. Update on antibacterial and antifungal drugs. Can we master the resistance crisis? Curr. Opin. Pharmacol., 11: 429–432. https://doi.org/10.1016/j.coph.2011.08.002
Walsh, T. and M. Toleman. 2012. The emergence of pan-resistant gram-negative pathogens merits a rapid global political response. J. Antimicrob. Chemother., 67: 1–3. https://doi.org/10.1093/jac/dkr378
Wayne, P., 2014. Performance standards for antimicrobial susceptibility testing. Twenty-Four Clinical and Laboratory Standards Institute.
Wiegand, I., K. Hilpert and R.E. Hancock. 2008. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc., 3(2): 163-175. https://doi.org/10.1038/nprot.2007.521
Zaman, S.B., M.M. Hussain, R. Nye, V. Mehta, K.T. Mamun and N. Hossain. 2017. A review on antibiotic resistance: Alarm bells are ringing. Cureus, 9(6): 1-9. https://doi.org/10.7759/cureus.1403
To share on other social networks, click on any share button. What are these?







