Evolutionary Adaptation of Fermenting Strains in Wheat Straw Hydrolysate for Bioethanol and Lactic Acid Production
Evolutionary Adaptation of Fermenting Strains Wheat Straw Hydrolysate for Bioethanol and Lactic Acid Production
Imrana Khushk1, Abdul Nabi Jatt2, Abdul Sattar Qureshi1*, Choudhary Haider Ali3, Muhammad Aqeel Bhutto1, Imran Ibrahim Khaskheli1, Sartar Iqbal Panhwer1
1Institute of Biotechnology and Genetic Engineering (IBGE), University of Sindh, Jamshoro (76080, Pakistan.
2 Institute of Microbiology, University of Sindh, Jamshoro (76080), Pakistan.
3Department of Chemical Engineering, University of Engineering and Technology, KSK Campus, Lahore (54890), Pakistan.
Abstract | Cellulosic biorefinery facing two major difficulties namely (pretreatment inhibitors and cost of cellulase enzyme). In the present study, long term evolutionary adaptation was evaluated for ethanol and lactic acid production to overcome the pretreatment inhibitors issue. Saccharomyces cerevisiae Angel was used for the production of ethanol, while, Bacillus coagulans, Pediococcus sp. and Lactobacillus sp. were used for lactic production, respectively. All the microbial strains were inoculated on wheat straw hydrolysate and were monitored with regular interval until stable cell growth, glucose consumption, and, ethanol and lactic acid production. Fermentation performance of the adapted strain improved over parental strains. Ethanol and lactic acid yield reached to 90% and 75%, respectively, when using freshly pretreated wheat straw or wheat straw hydrolysate or rice straw as carbon source. On-site produced cellulase from Bacillus subtilis OCS SH-2 was applied in the saccharification of pretreated wheat straw and rice straw. There was no significant difference when commercial cellulase youtell#7 and onsite produced cellulase were used in saccharification of high solid wheat straw simultaneous saccharification and fermentation (SSF). However, onsite produced cellulase does not need enzyme purification, packing and transportation. Hence, the production of bioethanol and lactic acid cost reduced at large extent by using on-site produced cellulase in the saccharification of lignocellulosic material.
Novelty Statement | In house cellulase production saved enzyme production, purification, and transportation cost. Evolutionary adapted strain produced higher ethanol and lactic acid titer from wheat straw. Adaptation of the fermenting strains reduce fermentation time and enhance the product yield
Article History
Received: December 21, 2020
Revised: March 03, 2021
Accepted: March 28, 2021
Published: June 10, 2021
Authors’ Contributions
IK performed experiments. ASQ, ANJ, MAB and IK wrote the manuscript. ANJ and ASQ analyzed the results. ASQ designed the study. CHA and MAB analyzed the data and revised the manuscript. IIK performed experiments of cellulase enzyme production. SIP performed lignocellulose pre-treatment experiments.
Keywords
Evolutionary adaptation, wheat straw hydrolysate, onsite cellulase, Bacillus subtilis OCS SH-2, freshly pretreated lignocellulosic biomass
Corresponding author: Dr. Abdul Sattar Qureshi
sattar.qureshi@usindh.edu.pk
To cite this article: Khushk, I., Jatt, A.N., Qureshi, A.S., Ali, C.H., Bhutto, M.A., Khaskheli, I.I. and Panhwer, S.I., 2021. Evolutionary adaptation of fermenting strainsin wheat straw hydrolysate for bioethanol and lactic acid production. Punjab Univ. J. Zool., 36(1): 47-56. https://dx.doi.org/10.17582/journal.pujz/2021.36.1.47.56
Introduction
Ethanol and lactic acid production based on lignocellulosic material is a promising strategy to develop alternative resources for increasing demands as fuel for transportation and biodegradable plastics. High lignocellulosic processing cost is the major hindrance in the commercialization of cellulosic biofuel and other commodity products (Lynd et al., 2008). Lignocellulosic biomass could be sustainable energy source for production of ethanol and commodity products. Evolutionary adaptation of ethanol producing strain Saccharomyces cerevisiae DQ1 was carried out to achieve increased ethanol titer and yield to decrease ethanol distillation energy cost (Qureshi et al., 2015b). Several research laboratories have tried to control cellulase cost by adopting different strategies including screening unique microbial strains with very high cellulase production rate, optimize fermentation conditions to further enhance cellulase concentration, using cost effective technique with inexpensive materials as carbon and nitrogen source, for example, agricultural wastes in solid substrate fermentation (SSF), characterization of the cellulase with tremendous saccharification properties. Low cost fermentation process, for example SSF that needs inexpensive materials (agricultural waste, fruit and vegetable waste) could be excellent choice for cellulase production with several advantages such as high enzyme production rate, reduce labor cost, reduce capital input and solid fermented material with cellulase enzyme could directly be applied in the saccharification step (Singhania et al., 2015).
Another difficulty of pretreatment inhibitors could be solved by long term evolutionary adaptation of the fermenting strain in hydrolysate media (Gu et al., 2014; Qureshi et al., 2015b). Long term adaptation enables microorganism to grow in harsh conditions, such as high temperature and inhibitors rich environments, and also improve the ethanol fermentability and inhibitors tolerance. This happens perhaps due to random mutation in certain genes. Inhibitors tolerance and fermentability of strain largely depends on the specific hydrolysate medium or solid feedstock in which it is grown. These ethanol and lactic acid fermenting strains have never experienced evolutionary adaptation towards pretreatment inhibitors, and a well-adapted strain will improve ethanol fermentability in high contents SSF resulting for the highest ethanol concentration and yield.
The search for ethanologenic microorganisms that are both inhibitor-tolerant and robust appears to be promising. Genetic engineering and long-term adaptation (direct evolution) are the two commonly used methods to obtain the inhibitory tolerant strains. From the current point of view, the microorganisms developed by genetic engineering or metabolic engineering demonstrate strong viability and good fermentation performance in certain harsh environments (mostly in the synthetic medium supplemented with inhibitors) but could not exhibit the same well in the real lignocellulose hydrolysate or SSF system (Cakar et al., 2005; Heer and Sauer, 2008).
On the other hand, long-term adaption mostly uses the real lignocellulose hydrolysate (with all the potential inhibitors present) and allows the microorganisms to adapt the hydrolysate environments gradually, concurrently causing the relevant-gene mutation or the complex inhibitory-tolerant mechanisms in the process. The obtained satisfactory strains always show robust inhibitory tolerance and have a higher ethanol fermentability. Heer and Sauer (2008), evolved the strain S. cerevisiae TMB3400 using inhibitors of the enhanced tolerance (furfural and HMF) through continuously transferring cells from previous fermentation medium (synthetic medium) to fresh medium. In adaptation medium furfural concentration was shown to be increased gradually and adaption was continued for over 300 generations (Heer and Sauer, 2008). The lag phase in the adapted strain was reduced to 17 h compared to parental strain with 90 h when synthetic medium was supplemented with 17mM furfural. Hawkins and Doran-Peterson (2011), investigated the evolutionary adaptation of industrial Saccharomyces yeast in the sulfur dioxide-pretreated pine wood system and found the improved fermentation performance in high solids loading SSF. Landaeta et al. (2013), investigated evolutionary adaptation of a flocculent strain of S. cerevisiae by growing in fermentation media with the increasing concentration of pretreatment inhibitors simulating to lignocellulosic hydrolysates during 39 consecutive days, and obtained the dramatically improved ethanol production and growth rate of 10% and 70% respectively, over a parent strain. Gu et al. (2014), proposed new strategy with evolutionary changes using synthetic medium to grow S. cerevisiae DQ1 with gradual increase of corncob residue hydrolysate and attained a satisfactory final concentration of ethanol with 62.68 g/L and 55.7% of ethanol yield.
The efficiency of adaptation is highly dependent on environmental conditions, including carbon sources, nutrient content, inhibitory components etc., all of which are alternatively determined by pretreatment methods or substrates. S. cerevisiae DQ1 adapted in corncob residues (Gu et al., 2014), and in corn stover hydrolysate (Qureshi et al., 2015b) showed excellent performance in high solid loading SSF. S. cerevisiae Angel and Pediococcus sp, Lactobacillus sp and Bacillus coagulans adaptability in the dilute acid pretreated wheat straw system has never been investigated.
Adaptation efficiency of fermenting strains depends on the specific environmental conditions where these microbial strains grow and ferment. Thus, the target strain to be used for the specific lignocellulose feedstock should be matched closely in the evolutionary adaptation. A strain that has adapted in a particular medium containing inhibitors or hydrolysate or solid feedstock medium will not necessarily perform well in another hydrolysate medium with changes in any aspects, such as feedstock type, pretreatment method, conditioning (detoxification), or hydrolysis conditions. During evolutionary adaption under the stress of inhibitor compounds, the mutation ratio of the adaptive strains is increased to enhance the fitness to the inhibitor environment and expand its population. This change should correspond to the up-regulation and down- regulation of specific genes responsible for the inhibitor tolerance. From the purpose of practical applications, evolutionary adaptation is used to obtain the evolved strain with improved tolerance and ethanol fermentability in high solids content SSF (Almario et al., 2013; Dragosits and Mattanovich, 2013).
In this study, the strains for ethanol and lactic acid production were subjected to evolutionary adaptation in wheat straw hydrolysate, in high solid content SSF, to achieve the highest ethanol and lactic acid concentration and yield. In order to further improve the concentration and yield of ethanol and lactic acid, the microbial strains were expressed for the long-term evolutionary adaptation using pretreatment inhibitors (freshly prepared hydrolysate of wheat straw). An evolutionary adapted strain was applied for the saccharification and fermentation of the freshly prepared wheat straw solids without detoxification or water washing to achieve high ethanol and lactic acid titer and yield with low processing cost.
Materials and Methods
Raw material
Wheat straw and rice straw were obtained from local areas of the District Jamshoro, Sindh, Pakistan. Wheat straw and rice straw were collected and washed with water to remove dust particles, then biomass was dried in open air. The dilute acid pretreated rice straw contained 41.24% of glucan and 2.97 % of xylan and the dilute acid pretreated wheat straw contained 3.46% of Xylan and 44.56% of glucan as per two-step H2SO4 hydrolysis method (Sluiter, 2012). Pretreated biomass was kept at room temperature for further process.
Strains and enzymes
Saccharomyces cerevisiae Angel, ethanol producing yeast strain was purchased from local market, China. This strain was used as parental strain and in long term evolutionary adaptation experiments for ethanol production. Bacillus coagulans strain stored in our laboratory and two locally isolated strains, Lactobacillus and Pediococcus spp. were used for lactic acid production. The onsite cellulase was produced from Bacillus subtilis OCS Sh-2. This strain was isolated and identified from soil samples. The onsite produced cellulase enzyme with filter paper activity of 4.3 FPU/mL was analyzed according to the method described by Adney and Baker (1996).
Pretreatment
Wheat and rice straw were pretreated with dilute acid at moderate temperature as reported in literature (Gabhane et al., 2014). Briefly, dilute acid solution (5.0% w/w) and dry wheat straw or rice straw were mixed at a solid/liquid ratio of 1:1 (w/w) followed by pretreating for 60 min at 121 oC. At the end of pretreatment, no free waste water was generated.
Wheat straw hydrolysate preparation
The hydrolysate prepared by the enzymatic hydrolysis of wheat straw after dilute acid pretreatment was directly used for long-term adaptation of S. cerevisiae and lactic acid producing bacterial strains. Briefly, the freshly pretreated wheat straw was hydrolyzed with 15 FPU/g DM of Youtell # 7 cellulase at 15% solids loading for 48 h at 50oC and 150 rpm (Zhang et al., 2010). After the enzymatic hydrolysis, the slurry was centrifuged for solid/liquid separation at 11,215 g for 10 min. Subsequently, clear supernatants were separated and used for the adaptation process. The wheat straw hydrolysate contained (g/L) glucose 52.14, xylose 15.37, acetic acid 1.46, furfural 0.28 and 5-hydroxymethylfurfural (HMF) 0.19. Before use, the nutrient composition, including 2.0 g/L of KH2PO4, 1.0 g/L of (NH4)2SO4, 1.0 g/L of MgSO4 .7H2O and 1.0 g/L of yeast extract was supplemented in medium for ethanol fermenting strain and the pH was adjusted to 6.0. Whereas, nutrient composition, including tryptone 10.0 g/L, yeast extract 10.0 g/L, MnSO4∙H2O 0.25 g/L, C6H14N2O7 2.0 g/L, MgSO4∙7H2O 0.58 g/L, K2HPO4 2.0 g/L, C6H12O6.∙H2O 22.0 g/L, CH3COONa 5.0 g/L was supplemented in medium for lactic acid producing strains and the pH was adjusted to 6.5.
Long term adaptation of ethanol and lactic acid fermenting strains
Long term adaptation of S. cerevisiae Angel was determined by transferring microbial culture continuously to a pure wheat straw hydrolysate (100%). Primarily, S. cerevisiae was inoculated into a synthetic broth medium (20 mL) followed incubation for 18 h at 30 oC, pH 6.0 and shaking speed 150 rpm. The components of the synthetic medium were same as the nutrients in the hydrolysate except for the 20 g/L of glucose. Then the activated yeast seeds were transferred into the wheat straw hydrolysate with inoculation ratio of 10 % (v/v) and incubated with 150 rpm shaking for 12 h at 37 oC. The adapted yeast was cultured using fresh hydrolysate at a 12 h interval. After each transfer, sample was withdrawn and centrifuged, and clear liquid was used for HPLC analysis. This process lasted for 50 days until the fermentation performance level off. Similarly, three lactic acid fermenting strains including, Pediococcus sp, Lactobacillus and Bacillus coagulans were also activated and grown on wheat straw hydrolysate. The fermentation performance of the obtained long-term adapted strains were evaluated in synthetic medium, wheat straw hydrolysate and SSF, respectively.
Solid seeds culture
Solid seeds culture was prepared as reported in Qureshi et al. (2015a). Seed culture was prepared in four steps. Step 1 culturing the dry yeast powder on synthetic medium. 0.2 g of S. cerevisiae Angel was transferred to 20 mL of synthetic broth medium (as described elsewhere) and followed by incubation for 18 h at 37oC with shaking at 150 rpm. In step 2, culture (5.0 mL) from step 1 was transferred into fresh broth medium (50 mL) and incubated with shaking speed of 150 rpm at 37 oC for 12 h. In step 2, 5% (v/v) freshly pretreated biomass was used as carbon source and Youtell #7 at 15 FPU/g DM was added. In the step 3 and 4 solid loading was enhanced to 10% v/v all other conditions were same. From step 4 the whole suspension was used as seeds culture for final SSF ethanol production using freshly pretreated wheat straw.
Onsite cellulase production from Bacillus subtilis OCS SH-2
The identification of the bacterial strain was achieved based on Gram staining reaction, morphological characteristics and the biochemical tests (Jatt et al., 2018), followed by 16S rRNA analysis using PCR technique. Sequence of the identified bacterium (B. subtilis OCS SH-2) was submitted at NCBI data base with accession number of KJ510651. A 5.0% (v/v) bacterial culture of B. subtilis OCS SH-2 was inoculated in enzyme production medium followed by incubation in shaking incubator at 50°C for 72 h at pH 5.0 (Cellulase production and characterization paper is in preparation). Molasses was used as sole carbon source with yeast extract as nitrogen source. At the end of fermentation, samples were collected to determine filter paper activity, β-glucosidase and CM-cellulase activities according to the methods reported by Eveleigh et al. (2009), Ghose (1987) and Adney and Baker (1996). Measurement of the total protein was obtained according to Bradford method (Bradford, 1976). All the fermentation experiments were performed in triplicate.
Simultaneous saccharification and fermentation (SSF) for ethanol and lactic acid production
SSF was performed in the shake flask using dilute acid pretreated wheat straw as feedstock. Primarily, the SSF process was started with 06 h pre-hydrolysis at 50 oC followed by SSF with 78 h at 37 oC. The dosage of cellulase was 15 FPU/g DM and the pH level was controlled manually at 5.5 using 5 M NaOH or 2 M H2SO4. The parental strain of S. cerevisiae was added after completion of a simple three-step seed activation (Qureshi et al., 2015a). The samples were obtained at regular intervals for ethanol, glucose and cells viability determination. Ethanol and glucose were analyzed from centrifuged samples on HPLC. Whereas, cell viabilities checked as reported by Qureshi et al. (2015a). Lactic acid SSF process was started at two stages. Stage 1: Pre-hydrolysis was started with addition of the Youtell #7 cellulase into the tank at the dosage of 15 FPU/g DM and the bio-detoxified feedstock was fed gradually to maintain the liquid slurry into the flask to a final 20 % w/w solids content within 6 h at 50 °C, 150 rpm, 4.8 pH. Stage 2: SSF was started by decreasing temperature to 42 °C and inoculating seeds of the three-step adapted lactic acid producing strain into separate SSF system. The complete SSF process lasted for 78 h. The samples were taken at regular interval and were centrifuged for 5 min at 11,167g. The supernatants were stored frozen until further analysis. Plate count method was used to determine cell viability during SSF operation (Qureshi et al., 2015a).
Analysis
The measurement of cellulose and hemicellulose contents in wheat and rice straw was obtained using two-step H2SO4 hydrolysis method (Sluiter et al., 2012). The dilute acid pretreated wheat straw contained 44.56 % of glucan and 3.46 % of xylan and dilute acid pretreated rice straw contained 41.24 % of glucan and 2.97 % of xylan.
HPLC was used to determine glucose, ethanol, lactic acid, acetic acid, HMF and furfural in the obtained samples. HPLC was operated at 65 oC with 0.6 mL/min of 5 mM H2SO4 as the mobile phase. Ethanol yield was calculated as per equation given by Zhang and Bao (2012).
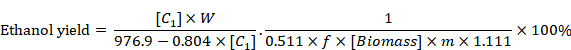
Where (C1) was the ethanol concentration in the fermentation medium (g/L), W was the total water used in SSF (g), f was the cellulose fraction of wheat straw, (Biomass) was the dry wheat straw concentration at the beginning of SSF (g/g), m was the total weight of SSF (g), 0.511 was the conversion factor for glucose to ethanol based on stoichiometric biochemistry of yeast, and 1.111 is the conversion factor for cellulose equivalent to glucose.
Lactic acid yield was calculated using the following equation as described in Zhao et al. (2013);

Where; (Lac)f and (Lac)0 were the lactic acid concentrations at the end and the beginning of the fermentation (g/L), Vf and V0 were the volume of liquid at the end and the beginning of the fermentation (L), W wheat straw was the weight of the dry wheat straw used in the SSF (g), f was the cellulose fraction of the dry wheat straw (g/g), 1.111 was the conversion factor for cellulose to equivalent glucose, 1.0 was the conversion factor for glucose to lactic acid on the mass basis of stoichiometric biochemistry.
Results and Discussion
Long-term adaptation of ethanol and lactic acid fermenting strains to enhance their fermentability
The main objectives of the present study were to enhance ethanol and lactic acid fermentability and reduce the lignocellulosic biorefinery cost. Long term adaption of ethanol fermenting strain by exposing it to lignocellulose derived inhibitors is an effective strategy to increase its inhibitor tolerance and fermentability (Heer and Sauer, 2008; Landaeta et al., 2013; Gu et al., 2014) In this study, S. cerevisiae Angel ethanol fermenting strain, Pediococcus sp, Lactobacillus sp and Bacillus coagulans lactic acid fermenting strains were subjected to long term adaptation by using wheat straw hydrolysate prepared by enzymatic hydrolysis of freshly pretreated wheat straw at 15% (w/w) solids loading. The adaption procedure of transferring cells from previous fermentation medium into the fresh wheat straw hydrolysate every 12 h was repeated until the fermentation performance reached a high and stable level. The glucose utilization and ethanol production or lactic acid production were chosen as the monitor criterion of microbial cells adaptation. As shown in Figure 1a, glucose consumption and ethanol production fluctuated around every 20 batches in the first 45 transfers. While after the 50th transfer, glucose in the hydrolysate was consumed out and the ethanol yield reached nearly 90 %. It is worth noting that both the glucose consumption and ethanol production were kept at a stable level after 50th transfers. In the case of lactic acid fermentation, Pediococcus sp. showed fluctuations up to 70 transfers, then the strain showed stability in terms of glucose consumption and lactic acid production (Figure 1b).
Figure 1c shows the evolutionary adaptation results of Lactobacillus sp in wheat straw hydrolysate. Strain showed fluctuation in glucose utilization and lactic acid production up to 65 transfers and then lactic acid production slightly increased probably due to well adaptation of strain to pretreatment inhibitors. Figure 1d shows the adaptation profile of B. coagulans in pure hydrolysate medium. In this case, strain showed instability in earlier transfer, but after 50th transfer glucose utilization and lactic acid production was stable. Evolutionary adaptation shows great potential of all used strains to adapt well in presence of pretreatment inhibitors that provides practical approach of lignocellulosic biomass utilization for biocommodities production. Landaeta et al. (2013) performed evolutionary adaptation of flocculating strain Saccharomyces cerevisiae NRRL Y-265 in synthetic medium containing inhibitory compounds simulating the lignocellulosic composition. Adaptation was repeated for 39 days then adapted strain was compared with parental strain and growth rate were 10% and 70% for parental and adapted strains. Adapted strain showed higher growth rate that is the function of adaptation. Gu et al. (2014) also suggest that evolutionary adaptation is better approach to reduce fermentation time and enhance ethanol production. They also performed adaptation of Saccharomyces cerevisiae DQ 1 in the corn cob residue. Our results also suggest that adaptation improves the fermentability of ethanol and lactic acid producing strains. Adapted strains produce higher concentration of the desired product in less time.
Evaluation of fermentation performances in synthetic medium, in wheat straw hydrolysate (SSF), and freshly pretreated wheat straw and rice straw
The fermentability of the adapted strains was evaluated in terms of pure glucose (synthetic medium), wheat straw hydrolysate and freshly pretreated wheat straw and rice straw, respectively. The results in Figure 2a showed that when the synthetic medium was used, there was no significant difference in ethanol titer and glucose consumption by both strains. Slightly improved growth by adapted strain over parental strain was observed. There was no difference in ethanol fermentability due to absence of inhibitors in the synthetic medium. In the case of wheat straw hydrolysate, major difference in ethanol production was observed. The ethanol was significantly high in the adapted strain as compared to the parental strain (22.8 g/L and 4.42 g/L, respectively). Qureshi et al. (2015b), showed that the adapted strain of S. cerevisiae DQ1 produced very high ethanol titer when compared to parental strain in hydrolysate of corn stover. In this study, SSF of the freshly pretreated wheat and rice straw (10% w/w solids) loaded with parental and adapted S. cerevisiae were also compared. The results shown in Figure 2a indicated that the consumption of glucose and the rate of ethanol production were greatly improved and this was accompanied by high levels of yeast cell viability when using the adapted yeast cells. Ethanol titer and yield were 24.2 g/L and 90.69%, respectively with the adapted yeast compared to parental strain (10.5 g/L corresponding to 38.90% yield) using freshly pretreated lignocellulosic biomass (without biodetoxification or water washing). In the case of lactic acid producing strains, the adapted strains performed better than parental strain in all fermentation medium with different carbon sources (synthetic medium, wheat straw hydrolysate, solid wheat straw or rice straw). Superior effect of the adapted strain was largely due to function of adaptation as shown in Figure 2b-d. In the solid wheat straw and rice straw medium, lactic acid titer reached to 34.1 g/L and 32.2 g/L corresponding to 75.62 % and 75.32 % yield when adapted B. coagulans was used compared to parental strain with 15.2 g/l and 12.5 g/L, respectively, corresponding to 31.32% and 26.80% yield (when 10% freshly pretreated wheat straw and rice straw were used). Results show that the adapted strains performed better compared to parental strains due to adaptation function of strains. SSF experiments were carried out to further confirm the behavior of strains to grow in freshly pretreated wheat straw without detoxification or water washing at high solids loading to achieve high ethanol and lactic acid titers. Utilization of freshly pretreated wheat straw reduce the detoxification step, save detoxification cost and reduces the amount of fresh water used to remove pretreatment inhibitors.
SSF performance with commercial cellulase youtell #7 and onsite produced cellulase
The lignocellulosic biorefinery faces some challenges, the most significant of which are cellulase production costs and dosage. Pretreated lignocellulosic biomass contains a large portion of cellulose that could be saccharified using one of the following strategies acid or alkali treatment and commercial enzyme cocktails and cellulase that is one of the major lignocellulolytic enzyme. Though commercial cellulases of Youtell, Novozymes and Gene core are available for saccharification of lignocellulosic materials but lignocellulosic biorefinery needs indigenous cellulolytic enzymes production unit to ensure continuous enzyme supply. Therefore, searching potential strains with higher cellulase production rate are need of the day and cellulase with high efficacy saccharification for lignocellulosic biorefinery application (Singhania et al., 2015). In this study, onsite cellulase production was carried out using B. subtilis OCS SH-2 in molasses medium. Crude onsite cellulase was applied to saccharify freshly pretreated wheat straw without washing or detoxification of the pretreated material. In order to obtain high ethanol titer and ethanol yield with the adapted yeast cells, the SSF process of the dilute acid pretreated wheat straw without biodetoxification was applied in SSF process. A 20% w/w of wheat straw was saccharified with youtell#7 cellulase and another set of experiment with onsite produced cellulase was used. Pre-hydrolysis of the freshly pretreated feedstock was conducted for 6 h using commercial and onsite produced crude cellulase then fermenting strain was inoculated to start real SSF process. 15 FPU/g DM cellulase was loaded in both set of experiments (Qureshi et al., 2015b). Moreover, it was observed that 35 g/L glucose was released in 6 h pre-hydrolysis when crude cellulase or commercial cellulase was applied. Ethanol concentration increased with passage of time and final ethanol concentration reached to 51.2 g/L of ethanol corresponding to 87.52% yield when 20% w/w freshly pretreated wheat straw was used. Cells viability in terms of cell forming unit (CFU) was also counted on agar plates as reported in Qureshi et al. (2015a). Cells viability for all fermenting strains increased with incubation time and ethanol or lactic acid concentration up to certain mark, then declined (data not shown).
There was no significant difference in ethanol titer and yield, as shown in Figure 3a. For lactic acid fermentation, 20% w/w wheat straw was saccharified only with onsite produced crude cellulase. Lactic acid titer reached to 60.1 g/L, 61.4 g/L and 63.4 g/L corresponding yield to 72.36%, 73.35%, and 76.44% when adapted strain Pediococcus sp, Lactobacillus sp and B. coagulans, respectively were used in fermentation of rice straw, results are shown in Figure 3b-d. Utilization of onsite produced cellulase in SSF of high solid loading will help in commercialization of biorefinery process for bioethanol and lactic acid production. Hoyer et al. (2013) obtained only 47.8 g/L of ethanol concentration corresponding to 72% ethanol yield at 25% solids content that was very low as compared to reported results 51.2 g/L of ethanol titer corresponding to 87.52% yield when 20% wheat straw was used. Tu et al. (2019) achieved 65.6 g/L corresponding to 69% yield using rice straw slurry. However, our result consistent with the previous study, and reported 63.4 g/L yield 76.44%. Thus, the current study has a clear advantage in terms of ethanol and lactic acid concentration and yield.
Conclusions and Recommendations
Ethanol producing strain of the yeast S. cerevisiae Angel and lactic acid producing strains Pediococcus sp, Lactobacillus sp, and B. coagulans were obtained by long term adaptation using wheat straw hydrolysate prepared from freshly pretreated wheat straw. By continuous exposure of ethanol fermenting strain to 100% wheat straw hydrolysate for 110 transfer number (12 h each), inhibitor tolerant strain with high fermentability rate was evolved. Similarly, exposure of lactic acid fermenting strains to 100% wheat straw hydrolysate for 100 transfer number (12 h each) improved lactic acid fermentability. When adapted strain of S. cerevisiae Angel was used in simultaneous saccharification and ethanol fermentation, it produced 51.2 g/L ethanol corresponding to 87.52 % yield under high solids loading 20% w/w of wheat straw. Lactic acid production reached to 63.4 g/L and 76.44 % yield when adapted B. coagulans was used. There was no significant difference when commercial cellulase youtell#7 and onsite produced cellulase were compared in saccharification of high solid wheat straw SSF. When on-site produced cellulase is used in the saccharification of lignocellulosic material then bioethanol and lactic acid production cost is reduced at large extent. Onsite produced cellulase does not need enzyme purification, packing and transportation.
Conflict of interest
The authors have declared no conflict of interest.
References
Adney, B. and Baker, J., 1996. Measurement of cellulase activities (LAP-006). NREL National Renewable Energy Laboratory, Golden.
Almario, M.P., Reyes, L.H. and Kao, K.C., 2013. Evolutionary engineering of Saccharomyces cerevisiae for enhanced tolerance to hydrolysates of lignocellulosic biomass. Biotechnol Bioeng., 110: 2616-2623. https://doi.org/10.1002/bit.24938
Bradford, M.M., 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 72: 248-254. https://doi.org/10.1016/0003-2697(76)90527-3
Çakar, Z.P., Seker, U.O.S., Tamerler, C., Sonderegger, M. and Sauer, U., 2005. Evolutionary engineering of multiple-stress resistant Saccharomyces cerevisiae. FEMS Yeast Res., 5: 569–578. https://doi.org/10.1016/j.femsyr.2004.10.010
Dragosits, M. and Mattanovich, D., 2013. Adaptive laboratory evolution-principles and applications for biotechnology. Microb. Cell Fact., 12: 64. https://doi.org/10.1186/1475-2859-12-64
Eveleigh, D.E., Mary, M., Raymond, A. and Charles, R., 2009. Measurement of saccharifying cellulase. Biotech. Bioful., 2: 21. https://doi.org/10.1186/1754-6834-2-21
Gabhane, J., William, S.P., Gadhe, A., Rath, R., Vaidya, A.N. and Wate, S., 2014. Pretreatment of banana agricultural waste for bio-ethanol production: Individual and interactive effects of acid and alkali pretreatments with autoclaving, microwave heating and ultrasonication. Waste Manage., 34: 498-503. https://doi.org/10.1016/j.wasman.2013.10.013
Ghose, T.K., 1987. Measurement of cellulase activities. Pure App. Chem., 59: 257-268. https://doi.org/10.1351/pac198759020257
Gu, H., Zhang, J. and Bao, J., 2014. Inhibitor analysis and adaptive evolution of Saccharomyces cerevisiae for simultaneous saccharification and ethanol fermentation from industrial waste corncob residues. Biores. Tech., 157: 6-13. https://doi.org/10.1016/j.biortech.2014.01.060
Hawkins, G.M. and Doran-Peterson, J., 2011. A strain of Saccharomyces cerevisiae evolved for fermentation of lignocellulosic biomass displays improved growth and fermentative ability in high solids concentrations and in the presence of inhibitory compounds. Biotechnol. Biofuel., 4: 49. https://doi.org/10.1186/1754-6834-4-49
Heer, D. and Sauer, U., 2008. Identification of furfural as a key toxin in lignocellulosic hydrolysates and evolution of a tolerant yeast strain. Microb. Biotechnol., 1: 497-506. https://doi.org/10.1111/j.1751-7915.2008.00050.x
Hoyer, K., Galbe, M. and Zacchi, G., 2013. The effect of prehydrolysis and improved mixing on high-solids batch simultaneous saccharification and fermentation of spruce to ethanol. Process Biochem., 48: 289-293. https://doi.org/10.1016/j.procbio.2012.12.020
Jatt, A.N., Tunio, S.A., Memon, S.B., Qureshi, A.S. and Bhutto, M.A., 2018. API-ZYM enzymatic profile of Shigella dysenteriae IM isolated from drinking water. Pak. J. Zool., 50: 977-981. https://doi.org/10.17582/journal.pjz/2018.50.3.977.981
Landaeta, R., Aroca, G., Acevedo, F., Teixeira, J.A. and Mussatto, S.I., 2013. Adaptation of a flocculent Saccharomyces cerevisiae strain to lignocellulosic inhibitors by cell recycle batch fermentation. Appl. Energ., 102: 124–130. https://doi.org/10.1016/j.apenergy.2012.06.048
Lynd, L.R., Laser, M.S., Bransby, D., Dale, B.E., Davison, B., Hamilton, R., Himmel, M., Keller, M., McMillan, J.D., Sheehan, J. and Wyman, C.E., 2008. How biotech can transform biofuels. Nat. Biotech., 26: 169-172. https://doi.org/10.1038/nbt0208-169
Qureshi, A.S., Zhang, J. and Bao, J., 2015a. Cellulosic ethanol fermentation using Saccharomyces cerevisiae seeds cultured by pretreated corn stover material. Appl. Biochem. Biotech., pp. 1-11. https://doi.org/10.1007/s12010-015-1480-y
Qureshi, A.S., Zhang, J. and Bao, J., 2015b. High ethanol fermentation performance of the dry dilute acid pretreated corn stover by an evolutionarily adapted Saccharomyces cerevisiae strain. Biores Technol., 189: 399-404. https://doi.org/10.1016/j.biortech.2015.04.025
Singhania, R.R., Saini, R., Adsul, M., Saini, J.K., Mathur, A. and Tuli, D., 2015. An integrative process for bio-ethanol production employing SSF produced cellulase without extraction. Biochem. Eng. J., 102: 45-48. https://doi.org/10.1016/j.bej.2015.01.002
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D. and Crocker, D., 2012. Determination of structural carbohydrates and lignin in biomass. In: LAP NREL/TP-510-42618. National Renewable Energy Laboratory, Golden CO.
Tu, W.L., Hsu, T.C., Wang, C.A., Guo, G.L. and Chao, Y., 2019. Using novel Lactobacillus plantarum to produce lactic acid from lignocellulosic biomass in an integrated simultaneous saccharification and fermentation process. Bioresources, 14: 3873-3885.
Zhang, J. and Bao, J., 2012. A modified method for calculating practical ethanol yield at high lignocellulosic solids content and high ethanol titer. Bioresour. Technol., 116: 74-79. https://doi.org/10.1016/j.biortech.2012.03.100
Zhang, J., Chu, D., Huang, J., Yu, Z., Dai, G. and Bao, J., 2010. Simultaneous saccharification and ethanol fermentation at high corn stover solids loading in a helical stirring bioreactor. Biotechnol. Bioeng., 105: 718–728. https://doi.org/10.1002/bit.22593
Zhao, K., Qiao, Q., Chu, D., Gu, H., Dao, T.H., Zhang, J. and Bao, J., 2013. Simultaneous saccharification and high titer lactic acid fermentation of corn stover using a newly isolated lactic acid bacterium Pediococcus acidilactici DQ2. Bioresour. Technol., 135: 481-489. https://doi.org/10.1016/j.biortech.2012.09.063
To share on other social networks, click on any share button. What are these?







