The Influence of Dietary Cadmium on Changes in the Midgut Mass Related to the Mass of Gypsy Moth Larvae
The Influence of Dietary Cadmium on Changes in the Midgut Mass Related to the Mass of Gypsy Moth Larvae
Milena Vlahović*, Dragana Matić, Marija Mrdaković, Larisa Ilijin, Anja Grčić, Aleksandra Filipović, Jelica Lazarević and Vesna Perić-Mataruga
Department of Insect Physiology and Biochemistry, Institute for Biological Research, “Siniša Stanković”, National Institute of Republic of Serbia, University of Belgrade, Despot Stefan Blvd 142, Belgrade, Serbia.
ABSTRACT
Cadmium pollution is becoming an increasing problem, especially in parts of the world that have developed industries. To consider the potentially harmful effects of cadmium, we need to examine changes at all different levels of biological organization. The main goal of this study was to detect a possible change in the percentage of midgut mass relative to larval mass (PMM) and determine the plasticity of this trait and the correlations between midgut enzymes and PMM under stress conditions. Fourth-instar larvae were exposed to acute and chronic effects of two cadmium concentrations, 10 and 30 μg Cd/g dry food, as well as a three-day recovery from chronic treatments. PMM is also an indirect indicator of food consumption and was found to be significantly reduced compared to control in both acute effects and chronic treatment at 30 μg and its three-day recovery. The PMM reduction during acute treatments is a consequence of cadmium action, while in chronic treatment, the genetic factor (egg mass) plays a crucial role in the change of PMM. According to the index of plasticity, distinct phenotypes were not produced. Significant correlations were shown between PMM and trypsin (Tryp) and leucine aminopeptidases (LAP) at acute and chronic treatment with higher cadmium concentrations, while significant correlations between proteases and PMM were detected at lower metal concentrations (Acute10 and Chronic10 and 30 μg Cd/g dry food). In contrast to chronic treatment, egg masses respond more uniformly by reducing PMM during the short-term effect of cadmium. Finally, we can conclude that, as an addition to biochemical and molecular research, PMM can be used for studying the cadmium effects to gain a better insight into the state of the organism under stress conditions.
Article Information
Received 19 October 2021
Revised 10 May 2022
Accepted 08 June 2022
Available online 09 November 2022
(early access)
Published 19 December 2023
Authors’ Contribution
MV designed, coordinated and
participated in all phases of the project. DM, AG and AF collected insects, reared them in laboratory and treated
larvae with cadmium. MM and JL
processed data. LI and VPM collected
literature data and participated in
manuscript writing.
Key words
Cadmium treatments, Gypsy moth, Midgut mass, Correlations, Plasticity
DOI: https://dx.doi.org/10.17582/journal.pjz/20211019131007
* Corresponding author: minavl@ibiss.bg.ac.rs
0030-9923/2024/0001-0339 $ 9.00/0
Copyright 2024 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Cadmium is a metal of great public health significance. This carcinogen has the ability to accumulate in many organs as well as in food (vegetables, cereals, and starchy roots). Anthropogenic activities are the main source of cadmium in nature. Cadmium presence in drinking water is almost irrelevant compared to dietary exposure (WHO, 2019). Nowadays, thanks to anthropogenic activities, cadmium progressively affects ecosystems. It is, therefore, necessary to determine the toxic effect of xenobiotics at different levels of biological organization.
The gut is responsible for the secretion and synthesis of enzymes, digestion, absorption of nutrients, and detoxification. It can be a model organ in the analysis of the harmful effects of metals introduced through food. In addition, it is the major tissue for metal accumulation (Zhang et al., 2001; Vlahović et al., 2017). In insects exposed to cadmium, a decline in the length of the alimentary canal, histological modifications in the midgut (disruption of epithelial cells and presence of vacuoles) and disruption of the peritrophic membrane are detected (Wu et al., 2009; Osman et al., 2015). Many authors have shown ultrastructural changes in the midgut during cadmium exposure (Hopkin and Martin, 1983; Vandenbulcke et al., 1998; Rost-Roszkowska et al., 2020). Besides, cadmium presence in the gut can lead to mitochondrial swelling, which can affect energy metabolism (Hemelraad et al., 1990). Metals can cause variations in epithelial thickness in the invertebrate digestive system. Furthermore, apoptosis was detected in phytophagous insects exposed to heavy metals (Rodrigues et al., 2008). Rost-Roszkowska et al. (2019) showed that acute and chronic cadmium treatments trigger cell death processes in insects. In their study, non-specific autophagy was found to maintain homeostasis under conditions of short-term exposure to metal (Rost-Roszkowska et al., 2018, 2019).
So far, we have found a small number of experiments that describe alterations in the midgut of phytophagous insects in the presence of cadmium. Changes in the morphological characteristics of organisms have long served as indicators of environmental quality (Lagisz, 2008). This type of analysis can complement biochemical and physiological biomarkers in environmental monitoring. Furthermore, beetles inhabit various biogeographical regions, have highly adaptive characteristics and are inexpensive, which is why there are numerous model systems available with well-described life cycles. Consequently, beetles can be a favorable bioindicator in studies of environmental protection.
We aimed to determine whether larvae of Lymantria dispar (Lepidoptera: Erebidae) exposed to chronic and acute dietary cadmium treatments have decreased midgut mass compared to the mass of the whole larvae. In the present paper, we focused on (1) midgut mass alteration in relation to the mass of the whole body (PMM); (2) plasticity of the examined trait during different cadmium treatments; (3) correlations between PMM and activities of midgut enzymes; (4) possible use of this parameter (PMM) as a biomarker of cadmium exposure.
MATERIALS AND METHODS
Experimental procedures
The egg masses of the gypsy moth analyzed in the experiment originate from a poplar forest. Twenty egg masses were collected at the Opovo site (45°03′07″ N and 20°25′49″ E). After collection, the 20 egg masses were held in the refrigerator at 4° C before placing in Petri dishes (volume 300 ml) for hatching. Larvae were reared at a density of 15 larvae per petri dish until the entry into the third larval stage when the density was reduced to five larvae/dish. Upon entering the fourth larval stage, the larvae were individually arranged in cups. The growing temperature was 23oC, with a 12h dark: 12h light photoperiod.
The larvae were reared on a high wheat germ diet (HWG), in which casein was replaced by yeast hydrolysate (Bell et al., 1981; O’Dell et al., 1984). Two cadmium concentrations were used: 10 g Cd/g dry food (further on referred to as 10) and 30 µg Cd/g dry food (designated as 30). Cadmium was added in the form of the nitrate salt: Cd (NO3)2 x 4H2O. Cadmium concentration in a diet was calculated with respect to its relative amount in the cadmium salt.
Experimental groups
Seven experimental groups were formed:
(i) Control a cadmium-free control group; (ii, iii) acute 10 and 30 acute treatment groups (larvae reared at 10 and 30 μg Cd/g dry food for three days of the fourth instar); (iv, v) chronic 10 and 30 chronic treatment groups (larvae reared at 10 and 30 μg Cd/g dry food from hatching until sacrificing); (vi, vii) recovery 10 and 30 recovery treatments (larvae reared at 10 and 30 μg Cd/g dry food until entering the fourth larval stage and then transferred to control for three days).
The analysis included 20 egg masses, 5 larvae per egg mass, and a total of 100 individuals within each experimental group (700 larvae in total). On the third day of the fourth larval instar, the larvae were measured and sacrificed. The abdominal cavity was opened, the midgut was separated from the foregut and hindgut and measured. All experimental procedures were in compliance with the Directive 2010/63/EU on the protection of animals used for experimental and other scientific purposes and were approved by the Ethical Committee for the Use of Laboratory Animals of the Institute for Biological Research “Siniša Stanković” National Institute of Republic of Serbia, University of Belgrade (Approval No. 54/10).
Calculation of the PMM
The percentage of the midgut mass (PMM) related to the mass of the larva on the third day of the fourth larval stage was calculated with the formula:
PMM(%) = (Mmidgut/Mlarvae) x100.
This parameter gives us an insight into the degree of food consumption in different environments (Jindra and Sehnal, 1989).
Enzyme activities as well as detection methods are described in our previous papers (Vlahović et al., 2008, 2014, 2015, 2017).
Statistical calculations
Statistical processing of the results was done with the computer program STATISTICA version 4.5 and SAS (edition 9.1.3., Service Pack 4, SAS Institute Inc, Covy, NC, USA, 2002-2003).
Analysis of variance was used to examine the significance of the effects of cadmium treatment. It was performed on log-transformed values of the examined traits. Norms-of-reaction plots show the plasticity of the response of PMM from different egg masses to the presence of different cadmium treatments.
The coefficient of phenotypic plasticity was calculated according to Cheplik (1995) using the following formula:
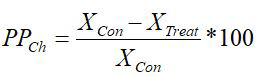
where PPCh is the index of phenotypic plasticity according to Cheplik and Xcon, XTr are the PMM at the control and treatment, respectively.
Wilcoxon’s test is used to compare the phenotypic plasticity indexes of different traits between different treatments. The significance of differences in index variation was determined by the F-test. The z-test was employed to compare correlation coefficients between different environments.
RESULTS
Figure 1 presents the mean values of the percentage of midgut weight at different treatments, while the significance of the changes was assessed by the LSD test. The percentage of gut mass decreases significantly during acute treatment at both cadmium concentrations. However, during the chronic effect of cadmium, the percentage of gut mass compared to the control decreases significantly only on 30 μg Cd/g dry food. In contrast to the recovery 10 group, where the percentage of gut mass did not differ significantly from the control, recovery from higher cadmium concentrations showed a significant decrease in the examined trait compared to control – no recovery occurred. A decrease in the PMM is an indirect indicator of reduced intake of contaminated food. The norm of reaction (lines-of-reaction norms) is a term that places phenotypic plasticity in the context of genotype-specific response. It shows an array of phenotypes that are developed by a genotype in different environments. The norms of reaction in Figure 2 show great variability in response to the same type of stress. In relation to control, in 13 egg masses, PMM decreased at Acute10, while at Acute 30, PMM decreased in 15 egg masses. During the recovery from 30 μgCd/g dry food, in as many as 15 egg masses, the PMM remained reduced to the level detected during chronic treatment (irreversible reduction).
Two-way ANOVA showed that cadmium significantly affects the change in the mean value of the PMM, P<0.01 (Table I) only in acute treatment. However, during the recovery from 30 μg Cd/g dry food, the genetic factor significantly contributes to the variation of the examined parameter, P<0.001.
The mean value of the index of phenotypic plasticity has only one negative value, namely, in chronic treatment at a lower concentration of cadmium (Table II). Comparison of the mean values of the phenotypic plasticity index and standard deviations of the PMM revealed there were no significant differences between the experimental groups, which means that the plasticity and its standard deviations cannot be taken as sensitive parameters to the presence of cadmium in food (Table II).
Table I. Mean squares (x103) from the two-way ANOVA of PMM in the gypsy moth larvae exposed to cadmium treatments; df, degrees of freedom, P < 0.05.
|
Treatments |
Variation |
df |
MS |
F |
P |
|
Acute |
Egg mass (EM) |
17 |
8.408 |
1.122 |
0.3351 |
|
Cadmium(Cd) |
2 |
53.657 |
6.430 |
0.0043 |
|
|
EMxCd |
34 |
8.345 |
1.113 |
0.3172 |
|
|
Error |
204 |
7.497 |
|||
|
Chronic |
Egg mass (EM) |
19 |
7.765 |
1.181 |
0.2749 |
|
Cadmium(Cd) |
2 |
13.437 |
1.417 |
0.2550 |
|
|
EMxCd |
38 |
9.485 |
1.442 |
0.0545 |
|
|
Error |
235 |
6.576 |
|||
|
Recovery10 |
Egg mass (EM) |
18 |
5.404 |
0.877 |
0.6072 |
|
Cadmium(Cd) |
2 |
3.342 |
0.381 |
0.6858 |
|
|
EMxCd |
36 |
8.769 |
1.423 |
0.0661 |
|
|
Error |
224 |
6.163 |
|||
|
Recovery30 |
Egg mass (EM) |
18 |
17.217 |
2.706 |
0.0003 |
|
Cad mium(Cd) |
2 |
23.321 |
3.158 |
0.0545 |
|
|
EMxCd |
36 |
7.385 |
1.161 |
0.2557 |
|
|
Error |
224 |
6.363 |
Statistically significant correlations were detected between PMM and (1) total proteases at Acute10 and both chronic cadmium treatments, (2) trypsin at chronic treatment with 30 μg Cd/g dry food as well as LAP at Acute 30 (Table III). The only negative correlation was found between PMM and total protease during short-term effect at 10 μg Cd/g dry food.
DISCUSSION
It is clear that a three-day regimen of cadmium primarily leads to a reduction in PMM, which is also proof that it is the organ first hit by xenobiotics in food. In addition, these changes were also detected after treatment with 30 μg Cd/g dry food. The decline of PMM is irreversible, as this parameter did not return to the control level even after the three-day recovery. Anthropogenic factors such as heavy metal contamination can lead to the selective survival of individuals of certain genotypes. In our experiment, statistically significant changes of PMM in acute treatments originate directly from cadmium, and the irreversible decline at chronic treatment is due to the influence of egg mass, i.e., genotype (ANOVA). The lines of reaction norms, representing individual variability per egg mass, show that egg masses respond much more uniformly by decreasing PMM during acute than during long-term treatment. In chronic cadmium treatment, there is greater variability. Genetic variability among different genotypes (20 egg masses) may give a diverse response to longer-lasting and stronger stress. The variability of population response to changes in the environment depends on genetic variability (Guttman, 1994; Templeton, 1995). However, it should be stressed that PMM is an indirect indicator of food consumption because the gut includes ingested food and not just tissue. We can conclude that cadmium in the fourth larval instar may lead to reduced food intake as well. It is likely that, for this trait, the concentration of 30 μg Cd/g dry food is too high to increase consumption, or a three-days regime is insufficient for recovery after chronictreatment. These findings are consistent with the
Table II. The mean values (MV) and standard deviations (±SD) calculated according to Cheplik (PPch) and the significance of differences between the mean values (Z-Wilcoxon’s test) and standard deviations (F-test) of the index of phenotypic plasticity.
|
10 μg Cd/g dry food |
30 μg Cd/g dry food |
|||||
|
N |
MV |
±SD |
N |
MV |
±SD |
|
|
Acute |
19 |
9.600 |
22.381 |
19 |
14.339 |
20.374 |
|
Chronic |
20 |
-5.871 |
24.429 |
20 |
3.245 |
19.858 |
|
Recovery |
19 |
1.726 |
19.908 |
19 |
11.666 |
14.617 |
|
Z |
P |
F |
P |
|||
|
Comparisons |
Acute10 |
-Acute30 |
1.2847 |
0.1989 |
1.2067 |
0.6944 |
|
Chronic10 |
-Chronic30 |
1.4933 |
0.1354 |
1.5133 |
0.3745 |
|
|
Recovery10 |
-Recovery30 |
1.8511 |
0.0642 |
1.8550 |
0.1996 |
|
|
Chronic10 |
-Acute10 |
1.7707 |
0.0766 |
1.1914 |
0.7139 |
|
|
-Recovery10 |
0.9256 |
0.3547 |
1.5057 |
0.3902 |
||
|
Chronic30 |
-Acute30 |
1.8511 |
0.0642 |
1.0526 |
0.9101 |
|
|
-Recovery30 |
1.2475 |
0.2122 |
1.8457 |
0.1997 |
||
Table III. Correlations between PMM and midgut enzymes during different cadmium treatments. Bold numbers represent correlations that are statistically significant P<0.05.
|
PMM |
α-glucosidase |
β- glucosidase |
Esterase |
Leucine aminopeptidase |
Trypsin |
Total protease |
Glutathione S-transferase |
|
Control |
0.0323 |
0.2026 |
-0.1769 |
-0.1876 |
-0.0334 |
-0.1373 |
-0.3718 |
|
Acute10 |
0.0376 |
0.4196 |
-0.5029+ |
-0.2698 |
-0.4163 |
-0.6288* |
0.1821 |
|
Acute30 |
0.0433 |
0.2715 |
0.0818 |
0.5430* |
0.3241 |
0.1405 |
-0.1642 |
|
Chronic10 |
0.2743 |
-0.2975 |
-0.1197 |
-0.0715 |
0.4042 |
0.4943* |
-0.2249 |
|
Chronic30 |
0.323 |
0.0311 |
0.1770 |
0.3244 |
0.5823* |
0.5970* |
0.0425 |
|
Recovery10 |
-0.0168 |
0.4366+ |
0.1126 |
0.1017 |
-0.1260 |
-0.0879 |
0.0315 |
|
Recovery30 |
-0.1609 |
-0.1273 |
0.0584 |
0.1118 |
0.2666 |
0.2330 |
-0.2034 |
results obtained by Fred and Brommer (2005), who found that larvae of two populations of Parnassius apollo reduced the intake of cadmium-contaminated food. It is possible that during long-term cadmium exposure, the chief priorities of individuals are survival and reaching adulthood. Therefore, all resources are directed towards proper development and thus adequate assimilation of food.
Restricted expression of enzymes may be associated with cadmium treatments as well as gut morphology. The decrease in midgut mass is accompanied by increased synthesis of enzymes that are crucial for survival in such conditions. There is probably a trade-off in the synthesis of digestive enzymes depending on which nutrients are most needed and/or available at the same time. In addition, under stress conditions, energy is redirected to the detoxification mechanisms that occur in the gut (sequestration, synthesis of metallothionein and oxidative enzymes, and the formation of granules containing metals).
All the enzymes that we claimed in our earlier researches (Vlahović et al., 2008, 2014, 2015, 2017) to be indicators of cadmium contamination show statistically significant correlations with PMM. The only negative correlation indicates different regulation of enzyme synthesis and/or activity in such conditions. Possibly, inhibition of protease isoforms leads to redistribution and preservation of homeostasis, while LAP and trypsin become the carriers of proteolytic function. In addition, during stronger cadmium stress (chronic treatment), a decrease in the activity of proteases and trypsin reduces PMM, the utilization of ingested food, and the survival rate. As previously stated (Vlahović et al., 2014, 2015), we have already detected the decline in digestive enzymes (LAP, TRYP and total PROT) during acute and chronic cadmium treatments. Several studies have suggested there are correlations between enzymatic activities in response to environmental stress or changes in food (Geer et al., 1985; Clark, 1989), and that correlated activities may result from simultaneous selection of the activity of several enzymes (Lande and Arnold, 1983). It can be concluded that, during cadmium presence, digestive enzymes can be mutually correlated and depend on environmental stress, the genetic basis, and most likely the structure of the enzyme itself.
Midgut structure influences enzymatic activity. The reason for this enzyme inhibition may be the altered structure and functioning of intestinal cells as a result of apoptosis and necrosis, which are direct consequences of cadmium action. Some ingested xenobiotics are detoxified via midgut epithelium (Johnson et al., 2009; Higes et al., 2013). Rost-Roszkowska et al. (2019) showed that acute and chronic cadmium treatments trigger the cell death process in Lithobius forficatus (Miriapoda). Non-specific autophagy of damaged organelles was mainly detected (Rost-Roszkowska et al., 2018, 2019). Reserve materials that occur in autophagosomes are utilized and removed from the gut as a way to diminish intracellular stress and reduce food intake (Wlodarczyk et al., 2019; Rost-Roszkowska et al., 2019). Short-term cadmium stress increases the metal concentration in the body. At the same time, ATP concentration is decreased regardless of the length of exposure to the metal. This can lead to reduced growth and changes in the structure and function of some organs (Rost-Roszkowska et al., 2020). Reduced amount of energy under stress conditions may affect the altered structure and function of the midgut in gypsy moth larvae. Degenerative necrotic changes in the midgut of the ground beetle are visible already after 1-2 days of cadmium exposure (Bednarska et al., 2016). With such substantial damage, cells are eliminated by necrosis, passive cell death. After metal exposure, apoptosis may be disturbed, and necrosis favored. Most likely, in our experiment, degeneration of the midgut epithelium resulted in reduced function and size of the midgut. Depending on the duration and severity of the treatment, the tissue damages may be irreversible (there is no recovery after the cadmium effect).
The degenerated gut epithelium is most probably unable to properly synthesize digestive enzymes, as seen in our previous work. Buchon et al. (2013) proved that in Drosophila melanogaster, most digestive gene families are organized in genomic clusters. Proteases, trypsins, α-esterases, mannosidases, and lipases form clusters, and their activation depends on where each of its members is located along the gut. A possible explanation for the selective enzyme inactivation may be the arrangement of enzymes into clusters in L. dispar larvae.
Due to all of the above, reduction of the alimentary canal can occur. The insects’ avoidance of contaminated food and inhibition of enzyme activities can be a consequence of the alterations in morphology and the changed functioning of the digestive system. In addition, under stress conditions, energy is redirected to the mechanisms of metal detoxification: The synthesis of metallothionein (Matić et al., 2020), synthesis of oxidative enzymes (Perić-Mataruga et al., 2019) as well as the formation of different granules (Hopkin, 1989). All these processes affect digestive enzymes and the utilization of nutrients in gypsy moth larvae. Thus, irreversible changes in fitness, metamorphosis and development arise, leading to reduced survival.
It is very important to note that the irreversible decline in larval mass begins only after the effect of a higher metal concentration upon chronic treatment at 30 μgCd/g dry food (Vlahović et al., 2014). During acute cadmium treatment, larval mass is constant, unlike reduced PMM. This phenomenon indicates that the gut is the first organ struck by the harmful effect of food toxicants and that its alteration does not have to be correlated with the size of the larvae.
Unlike midgut enzymes, statistically significant changes in the index of phenotypic plasticity or its standard deviations of the PMM between the treatments were not detected.
We demonstrated that metabolic and functional losses even at a low cadmium concentration, such as 10 μgCd/g dry food, which represents the NOEC for larval growth (Vlahović et al., 2001), can damage insects’ midgut. The digestive system is the first to react to the presence of cadmium in food. We can conclude that the synthesis of digestive enzymes is correlated with the size of the intestine and vice versa, and a comprehensive study of the effect of dietary cadmium should thus analyze both biochemical parameters and structural changes in the midgut. This rapid screening method can be used to demonstrate the harmful effects of xenobiotics in food in a fast and inexpensive way or may complement biochemical tests in environmental studies.
ACKNOWLEDGEMENTS
This work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (contract no. 451-03-68/2022-14/ 200007).
Statement of conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Bednarska, A.J., Laskowski, R., Pyza, E., Semik D., Świątek, Z., and Woźnicka, O., 2016. Metal toxicokinetics and metal-driven damage to the gut of the ground beetle Pterostichus oblongopunctatus. Environ. Sci. Pollut. Res., 23: 22047–22058. https://doi.org/10.1007/s11356-016-7412-8
Bell, R.A., Owens, C.D., Shapiro, M., and Tardif, J.R., 1981. Mass rearing and virus production: Development of mass-rearing technology. In: The gypsy moth: Research toward integrated pest management (eds. C.C. Doane and M.L. McManus). USDA, Forest Service, Technical Bulletins, pp. 1584.
Buchon, N., Osman, D., David, F.P., Fang, H.Y., Boquete, J.P., Deplancke, B., and Lemaitre, B., 2013. Morphological and molecular characterization of adult midgut compartmentalization in Drosophila. Cell Reprod., 3: 1725-1738. https://doi.org/10.1016/j.celrep.2013.04.001
Cheplik, G.P., 1995. Genotypic variation and plasticity of clonal growth in relation to nutrient availability in Amphibromus scabrivalvis. J. Ecol., 83: 459-468. https://doi.org/10.2307/2261599
Clark, A.G., 1989. Causes and consequences of variation in energy storage in Drosophila melanogaster. Genetics, 123: 131-144. https://doi.org/10.1093/genetics/123.1.131
Fred, M.S., and Brommer, J.E., 2005. The decline and current distribution of Parnassius apollo (Linnaeus) in Finland; the role of Cd. Ann. Zool. Fenn., 42: 69-79.
Geer, B.W., Langevin, M.L., and McKechnie, W.W., 1985. Dietary ethanol and lipid synthesis in Drosophila melanogaster. Biochem. Genet., 23: 607-622. https://doi.org/10.1007/BF00504295
Guttman, S.I., 1994. Population genetic structure and ecotoxicology. Environ. Hlth. Persp., 102 (Suppl. 12): 97-100. https://doi.org/10.1289/ehp.94102s1297
Hemelraad, J., Herwig, H.J., van Donselaar, E.G., Holwerda, D.A., and Zandee, D.I., 1990. Effects of cadmium in freshwater clams. II. Ultrastructural changes in the renal system of Anodonta cygnea. Arch. Environ. Contamin. Toxicol., 19: 691–698. https://doi.org/10.1007/BF01183986
Higes, M., Meana, A., Bartolome, C., Botias, C., and Martin-Hernandez, R., 2013. Nosema ceranae (microsporidia), a controversial 21st century honey bee pathogen. Environ. Microbiol. Rep., 5: 17–29. https://doi.org/10.1111/1758-2229.12024
Hopkin, S.P., 1989. Ecophysiology of metals in terrestrial invertebrates. Elsevier Applied Science, Elsevier, New York.
Hopkin, S.P., and Martin, M.H., 1983. Heavy metals in the centipede Lithobius variegatus (Chilopoda). Environ. Pollut., 6B: 309-318. https://doi.org/10.1016/0143-148X(83)90016-2
Jindra, M., and Sehnal, F., 1989. Larval growth, food consumption, and utilization of dietary protein and energy in Galleria mellonella. J. Insect Physiol., 35: 719-724. https://doi.org/10.1016/0022-1910(89)90091-7
Johnson, R., Evans, J.D., Robinson, G.E., and Berenbaum, M.R., 2009. Changes in transcript abundance relating to colony collapse disorder in honey bees (Apis mellifera). Proc. natl. Acad. Sci. U.S.A., 106: 14790–14795. https://doi.org/10.1073/pnas.0906970106
Lagisz, M., 2008. Changes in morphology of the ground beetle Pterostichus oblongopunctatus F. (Coleoptera; Carabidae) from vicinities of a zinc and lead smelter. Environ. Toxicol. Chem., 27:1744–1747. https://doi.org/10.1897/07-661.1
Lande, R., and Arnold, S.J., 1983. The measurement of selection on correlated characters. Evolution, 37: 1210-1226. https://doi.org/10.1111/j.1558-5646.1983.tb00236.x
Matić, D., Vlahović, M., Ilijin, L., Mrdaković, M., Grčić, A., Filipović, A., and Perić Mataruga, V., 2020. Metallothionein level, non-specific esterases, fitness-related traits and integrated biomarker response (IBR) in larvae of Lymantria dispar L. (Lepidoptera) originating from unpolluted and polluted locations after chronic cadmium treatment. Ecol. Indicat., 112: 106136. https://doi.org/10.1016/j.ecolind.2020.106136
O’dell, T.M., Bell, R.A., Mastro, V.C., Tanner, J.A., and Kennedy, J.F., 1984. Production of the gypsy moth, Lymantria dispar, for research and biological control. In: Advanced and challenges in insect rearing (eds E.G. King and N.C. Leppla). USDA-ARS, pp. 156-166. New Orleans, LA.
Osman, W., El-Samad, L.M., Mokhamer, el-H., El-Touhamy, A., and Shonouda, M., 2015. Ecological, morphological, and histological studies on Blaps polycresta (Coleoptera: Tenebrionidae) as biomonitors of cadmium soil pollution. Environ. Sci. Pollut. Res. Int., 22: 14104-14115. https://doi.org/10.1007/s11356-015-4606-4
Perić-Mataruga, V., Ilijin, L., Mrdaković, M., Todorović, D., Prokić, M., Matić, D., and Vlahović, M., 2019. Parameters of oxidative stress, cholinesterase activity, Cd bioaccumulation in the brain and midgut of Lymantria dispar (Lepidoptera: Lymantriidae) caterpillars from unpolluted and polluted forests. Chemosphere, 218: 416-424. https://doi.org/10.1016/j.chemosphere.2018.11.112
Rodrigues, A., Cunha, L., Amaral, A., Medeiros, J., and Garcia, P., 2008. Bioavailability of heavy metals and their effects on the midgut cells of a phytopaghous insect inhabiting volcanic environments. Sci. Total Environ., 406: 116-122. https://doi.org/10.1016/j.scitotenv.2008.07.069
Rost-Roszkowska, M.M., Janelt, K., and Poprawa, I., 2018. The role of autophagy in the midgut epithelium of Parachela (Tardigrada). Zoomorphology, 37: 501–509. https://doi.org/10.1007/s00435-018-0407-x
Rost-Roszkowska, M., Vilimová, J., Tajovský, K., ChachulskaŻymełka, A., Sosinka. A., Kszuk-Jendrysik, M., Ostróżka, A., and Kaszuba, F., 2019. Autophagy and apoptosis in the midgut epithelium of millipedes. Microsc. Microanal., 25: 1004–1016. https://doi.org/10.1017/S143192761900059X
Rost-Roszkowska, M., Poprawa, I., Chajec, Ł., Chachulska-Żymełka, A., Wilczek, G., Wilczek, P., Student, S., Skowronek, M., Nadgórska-Socha, A., and Leśniewska, M., 2020. Influence of soil contaminated with cadmium on cell death in the digestive epithelium of soil centipede Lithobius forficatus (Myriapoda, Chilopoda). Eur. Zool. J., 87: 242-262. https://doi.org/10.1080/24750263.2020.1757168
Templeton, A.R., 1995. Biodiversity at the molecular level: Experiences from disparate macroorganisms. In: Biodiversity: Measurement and estimation (ed. D.L. Hawksworth). Chapman and Hall, New York. pp. 59-64. https://doi.org/10.1098/rstb.1994.0086
Vandenbulcke, F., Grelle, C., Fabre, M-C., and Descamps, M., 1998. Implication of the midgut of the centipede Lithobius forficatus in the heavy metal detoxification process. Ecotoxicol. Environ. Saf., 4: 258–268. https://doi.org/10.1006/eesa.1998.1706
Vlahović, M., Ilijin, L., and Lazarević, J., 2001. Acute effect of cadmium on larval growth of Lymantria dispar L. Ekologija, 36: 132–137.
Vlahović, M., Lazarević, J., Perić-Mataruga, V., Ilijin, L., and Mrdaković, M., 2008. Plastic response of larval mass and alkaline phosphatase to cadmium in the gypsy moth larvae. Ecotoxicol. Environ. Saf., 72: 1148-1155. https://doi.org/10.1016/j.ecoenv.2008.03.012
Vlahović, M., Ilijin, L., Lazarević, J., Mrdaković, M., Gavrilović, A., Matić, D., and Perić Mataruga, V., 2014. Cadmium-induced changes of gypsy moth larval mass and protease activity. Comp. Biochem. Physiol. C, 160: 9-14. https://doi.org/10.1016/j.cbpc.2013.11.002
Vlahović, M., Ilijin, L., Mrdaković, M., Gavrilović, A., Matić, D., Lazarević, J., Perić-Mataruga, V., 2015. Alteration of the activities of trypsin and leucine aminopeptidase in gypsy moth caterpillars exposed to dietary cadmium. Water Air Soil Pollut., 226: 387-340. https://doi.org/10.1007/s11270-015-2651-8
Vlahović, M., Matić, D., Mutić, J., Trifković, J., Đurđić, S., and Perić Mataruga, V., 2017. Influence of dietary cadmium exposure on fitness traits and its accumulation (with an overview on trace elements) in Lymantria dispar larvae. Comp. Biochem. Physiol. C, 200: 27-33. https://doi.org/10.1016/j.cbpc.2017.06.003
WHO, 2019. Exposure to cadmium: A major public health concern.
Włodarczyk, A., Student, S., and Rost-Roszkowska, M., 2019. Autophagy and apoptosis in starved and refed Neocaridina davidi (Crustacea, Malacostraca) midgut. Can. J. Zool., 97: 294–303. https://doi.org/10.1139/cjz-2018-0104
Wu, G.X., Gao, X., Ye, G.Y., Li, K., Hu, C., and Cheng, J.A., 2009. Ultrastructural alterations in midgut and Malpighian tubules of Boettcherisca peregrina exposure to cadmium and copper. Ecotoxicol. Environ. Saf., 72: 1137–1147. https://doi.org/10.1016/j.ecoenv.2008.02.017
Zhang, Y., Lambiase, S., Fasola, M., Gandini, C., Grigolo, A., and Laudani, U., 2001. Mortality and tissue damage by heavy metal contamination in the German cockroach, Blattella germanica (Blattaria, Blattellidae). Ital. J. Zool., 68: 137-145. https://doi.org/10.1080/11250000109356398
To share on other social networks, click on any share button. What are these?